- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel A Level Chemistry:复习笔记1.1.3 Relative Mass
Isotopic & Atomic Mass
- The relative mass of an atom uses the carbon-12 isotope as the international standard
- One atom of carbon-12 has an accepted mass of 1.992646538 x 10-26 kg
- It is not realistic to work with this value so the mass of a carbon-12 atom is fixed as exactly 12 atomic mass units / 12υ
- The standard mass for atomic mass is 1υ
- Therefore, the standard mass for comparison is the mass of 1 ⁄ 12 of a carbon-12 atom
Relative isotopic mass
- Relative isotopic mass is defined as the mass of an isotope relative to 1 ⁄ 12 of a carbon-12 atom
- For A Level Chemistry it is common to work with mass values rounded to one decimal place, for example:
- The accurate relative isotopic mass of nitrogen is 14.00307401 but this is rounded to 14.0
- The accurate relative isotopic mass of oxygen is 15.99491464 but this is rounded to 16.0
Relative atomic mass
- Most elements on the Periodic Table represent a mixture of different isotopes, which is shown as their relative atomic mass (Ar)
- The relative atomic mass is the weighted mean / average mass of an atom relative to 1 ⁄ 12 of the mass of a carbon-12 atom
Molecular & Formula Mass
- We have seen previously that the symbol for the relative atomic mass is Ar
- This is calculated from the mass number and relative abundances of all the isotopes of a particular element
- The symbol for the relative formula mass is Mr and it refers to the total mass of the substance
- The term relative formula mass should be used for compounds with giant structures e.g. ionic compounds such as sodium chloride
- If the substance is molecular you can use the term relative molecular mass
- To calculate the Mr of a substance, you have to add up the relative atomic masses of all the atoms present in the formula
Relative Formula Mass Calculations Table
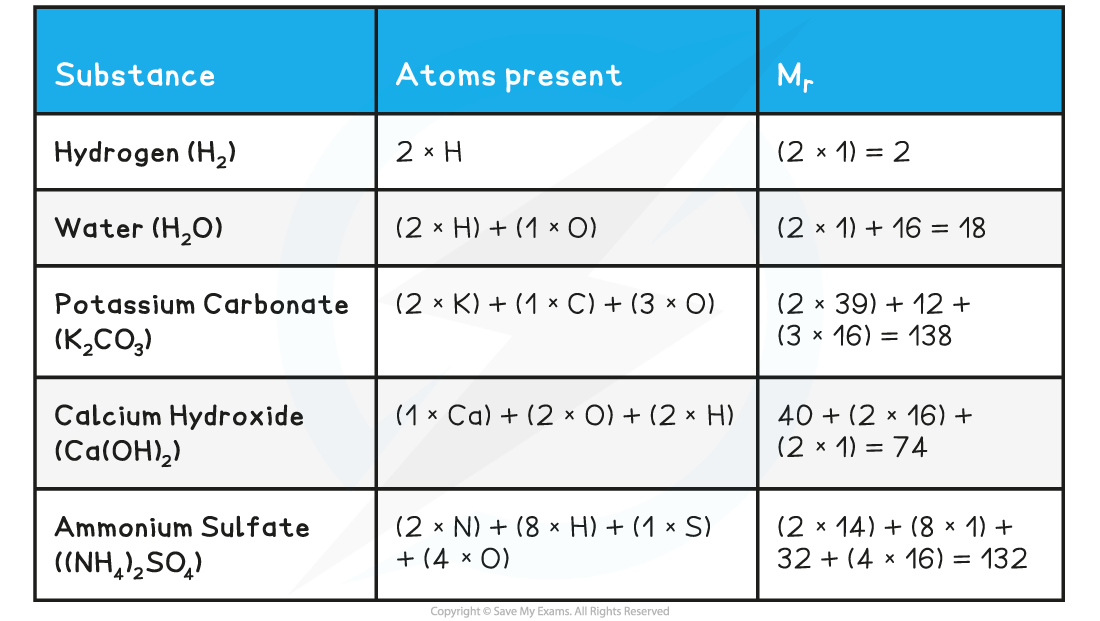
Exam Tip
It is expected that you will use relative atomic mass values from the Periodic Table
- This means that your values will be more accurate
- e.g. potassium carbonate = (2 x 39.1) + 12.0 + (3 x 16.0) = 138.2
If you are in any doubt whether to use relative molecular mass or relative formula mass, use the latter because it applies to all compounds whether they are ionic or covalent.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





