- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记8.1.3 Interpreting Rf Values in GL Chromatography
Interpreting Rf Values in GL Chromatography
Features of a gas-liquid chromatogram
- Peaks represent different molecules from the sample - each roughly taking the shape of a triangle
- The area under each peak is the relative concentration of each component (the peak integration value)
Area under the peak = ½ x base x height
- If the area under each peak is very small or too difficult to decipher, the height of peaks are used for further analysis
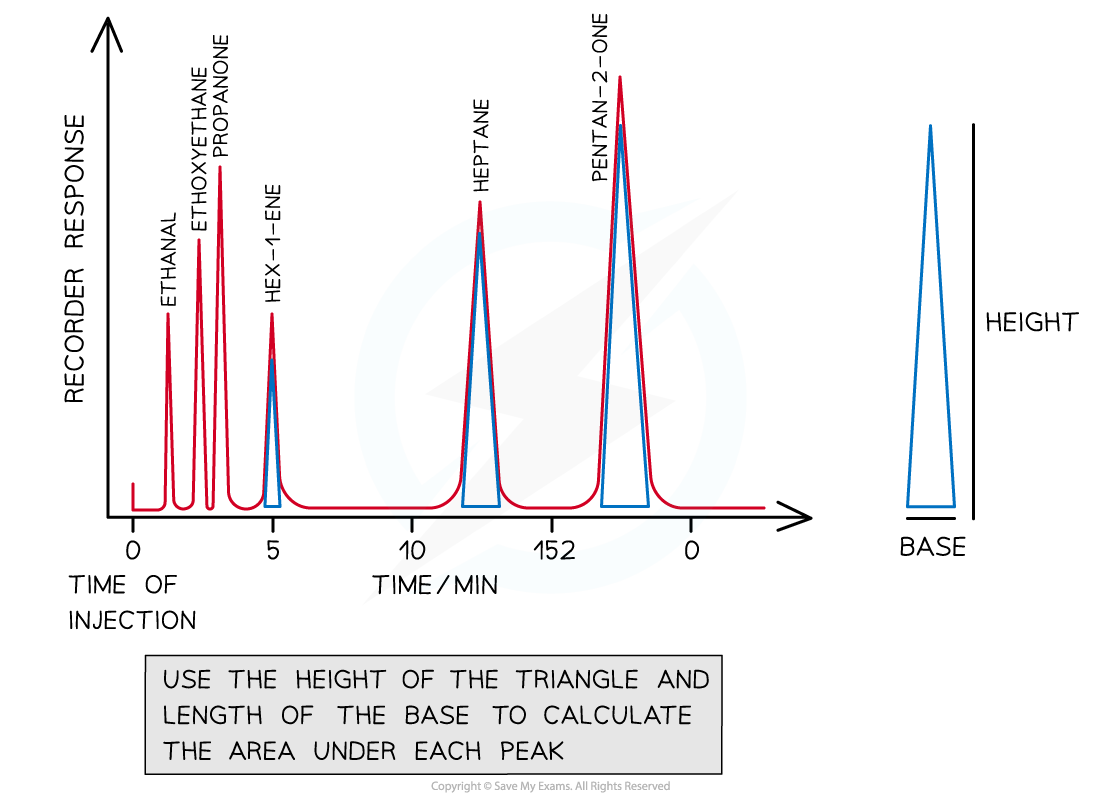
To find the area under each peak, treat each peak as a triangle - see the examples shown using blue triangles in the diagram
Percentage composition of a mixture
- We can calculate the amount of a particular molecule in a sample by using an expression
- If a chromatogram shows peaks for alcohols A, B, C and D, to calculate the % composition of alcohol C, use this expression:
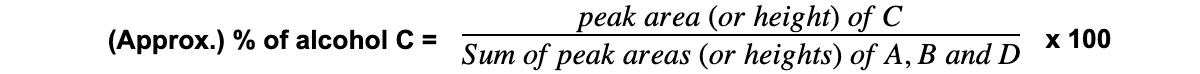
Explain Retention Times
- Retention time is the time taken for a sample molecule to travel through the column, from the time it is inserted into the machine to the time it is detected
- Molecules in the gaseous mixture travel at different rates, therefore giving rise to different retention times
- Longer retention times are associated with:
- Non-polar components in the mixture
- They are more attracted to the non-polar liquid in the stationary phase
- So non-polar molecules travel slower through the column
- Shorter retention times are associated with:
- Polar components in the mixture that prefer to interact with the carrier gas
- They are less attracted to the non-polar liquid in the stationary phase
- So polar molecules travel faster through the column
- These molecules may have lower boiling points, therefore are vapourised more readily
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





