- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记8.1.2 Gas/Liquid Chromatography: Basics
Gas/Liquid Chromatography: Basics
- Gas-Liquid Chromatography (GLC) is used for analysing:
- Gases
- Volatile liquids
- Solids in their vapour form
- The stationary phase:
- This method uses a column for the stationary phase
- A non-polar, long-chain, non-volatile hydrocarbon with a high boiling point is mounted onto a solid support
- Small silica particles can be packed into a glass column to offer a large surface area
- Sample gas particles travel through this phase and are able to separate well due to the large surface area
- The Mobile phase
- An inert carrier gas (eg. Helium, Nitrogen) moves the sample molecules through the stationary phase
Retention times
- Once sample molecules reach the detector, their retention times are recorded
- This is the time taken for a component to travel through the column
- The retention times are recorded on a chromatogram where each peak represents a volatile compound in the analysed sample
- Retention times are then compared with data book values to identify unknown molecules
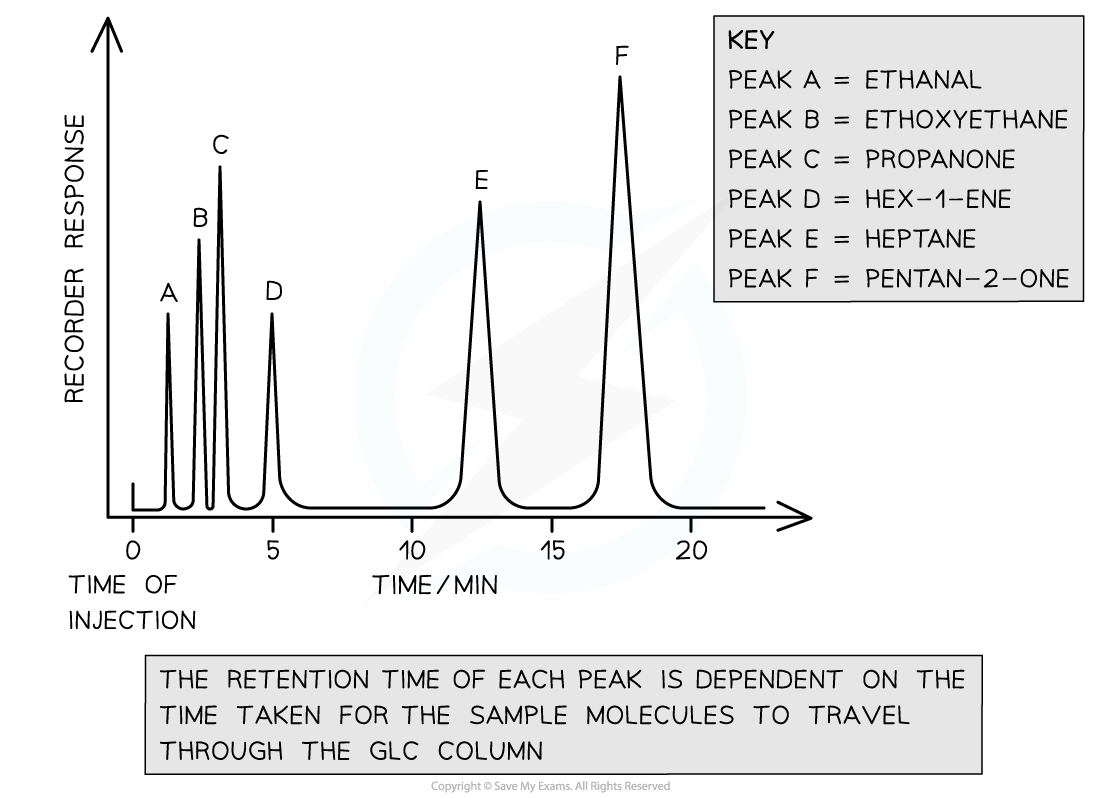
A gas chromatogram of a volatile sample compound has six peaks. Depending on each molecule’s interaction with the stationary phase, each peak has its own retention time
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





