- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.7.5 Degradabiity of Polymers
Poly(alkenes) & Biodegradability
- Many of the polymers in use have been produced through addition polymerisation of alkenes
- The (poly)alkene chains are non-polar and saturated
- This makes them chemically inert and therefore non-biodegradable
- (poly)alkenes can be melted and recycled into new uses
- However, even in the new applications, the (poly)alkenes are not biodegradable
- Recycling plants can burn used plastic materials
- The energy released from burning can be used to generate electricity
- Burning plastics in oxygen releases carbon dioxide and water (complete combustion) which can contribute to global warming
Photodegradation of Polymers
- Polyesters and polyamides are biodegradable polymers for a number of reasons
- One such reason is their ability to breakdown with the use of light
- Carbonyl groups (C=O) along polymer chains are able to absorb energy from the Electromagnetic Spectrum
- In particular Ultraviolet (UV) light
- Absorbing UV light weakens the carbonyl areas of polymers and breaks them down into smaller molecules
Disadvantages of photo degradability
- Despite this ability being a great advantage of polyesters and polyamides, it may pose a problems when the polymers are repurposed
- When applied to a new use, the biodegradability could give a weaker polymer
- Breaking down polymers also poses another challenge
- Once used, polymeric materials are taken to landfill sites where many other materials are piled on top of each other
- This could mean that photodegradable polyesters or polyamides do not have access to UV light in order to break down naturally
Biodegrading Polyesters & Polyamides
Biodegradable polymers
- Both polyesters and polyamides can be broken down using hydrolysis reactions
- This is a major advantage over the polymers produced using alkene monomers (polyalkenes)
- When polyesters and polyamides are taken to landfill sites, they can be broken down easily and their products used for other applications
Hydrolysis of polyamides
- Hydrolysis is a breaking up of a molecules using water
- In acidic hydrolysis, acid (such as hydrochloric acid) acts as the catalyst
- Polyamides are heated with dilute acid
- This reaction breaks the polyamide into carboxylic acid molecules and ammonium chloride ions
- Alkaline hydrolysis
- The polyamide is heated with a species containing hydroxide ions (eg. sodium hydroxide)
- This breaks the polymer into the sodium salts of its monomers (dicarboxylic acids and diamines)
- If the poly amide link used an aminocarboxylic acid as the monomer, then a sodium salt of the original amino acid is reformed
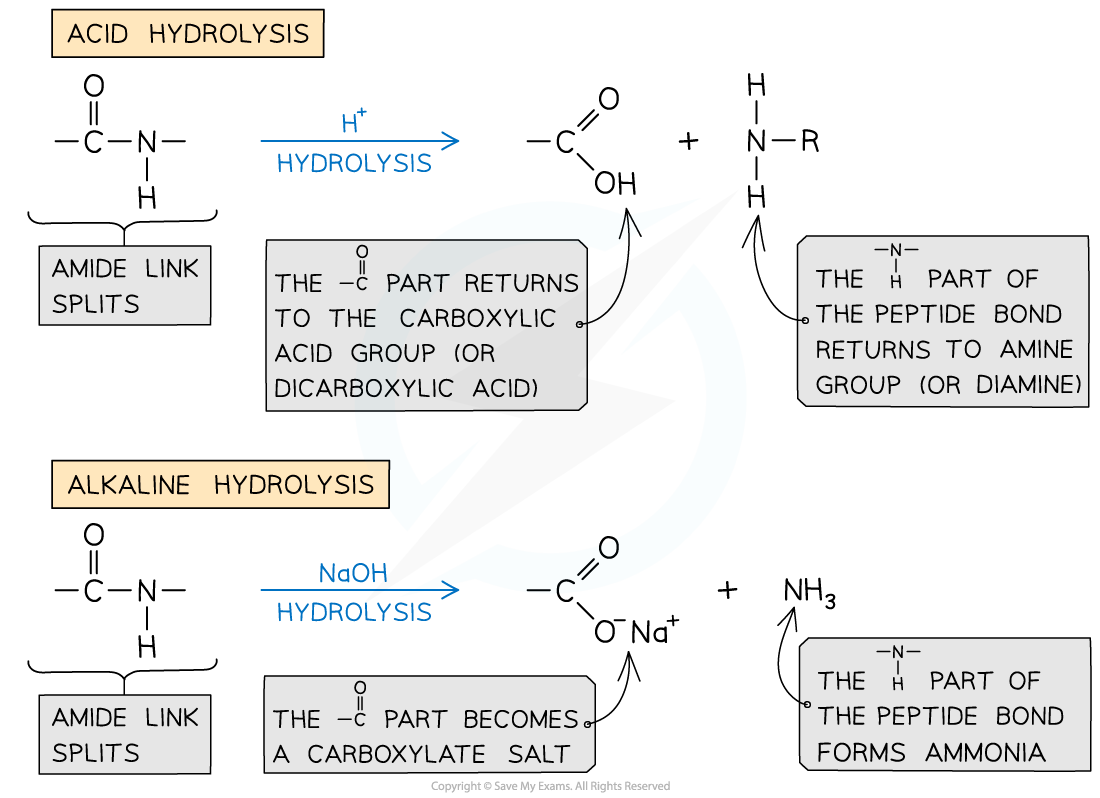
When polyamides are degraded by hydrolysis, carboxylic acids and amines are formed
Hydrolysis of polyesters
- Ester linkages can also be degraded through hydrolysis reactions
- Acid hydrolysis forms the alcohols and carboxylic acids that were used to form the polyesters
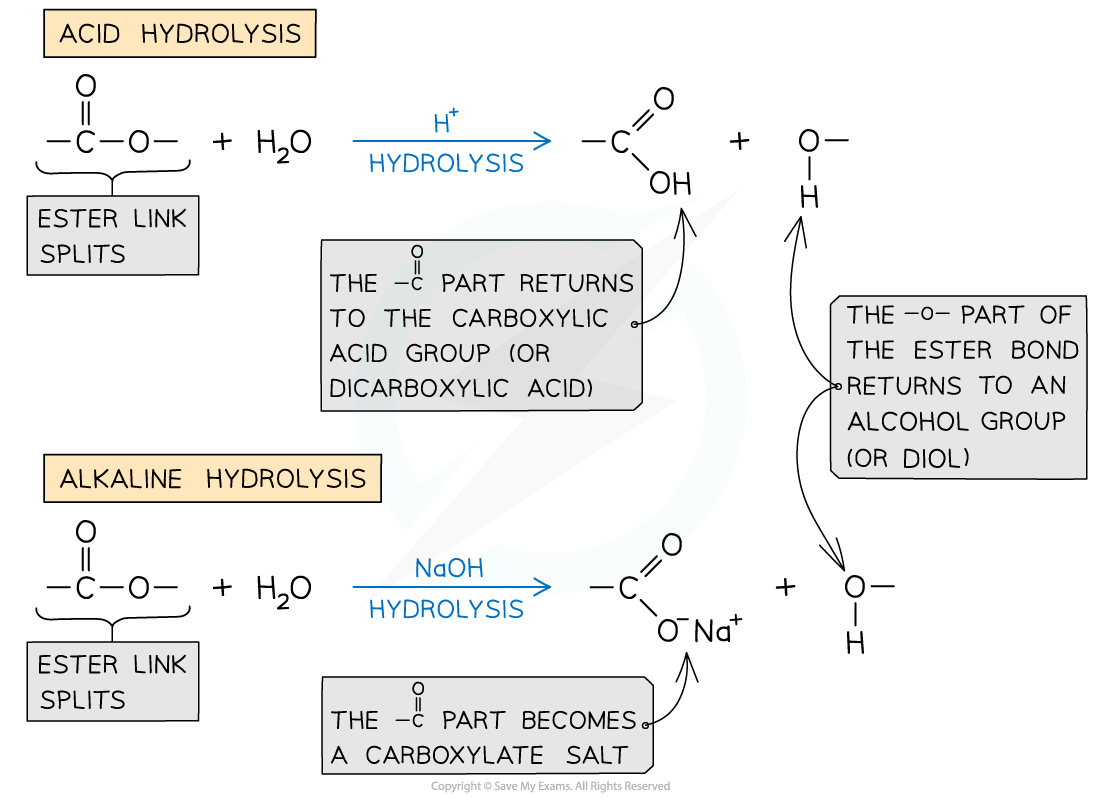
When polyesters are degraded by hydrolysis, carboxylic acids and alcohols are formed
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





