- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.7.4 Predicting & Deducing the Type of Polymerisation
Predicting Type of Polymerisation
- When a set of monomers are given in an exam question, the type of polymerisation can be determined
- Firstly, it’s important to identify the key functional groups in the monomers
Addition polymerisation
- If the monomer/s contain a C=C double bond, they will polymerise through addition polymerisation
- The double bond can open up in order to add more monomers either side of the starting monomer
- This type of polymerisation makes (poly)alkenes
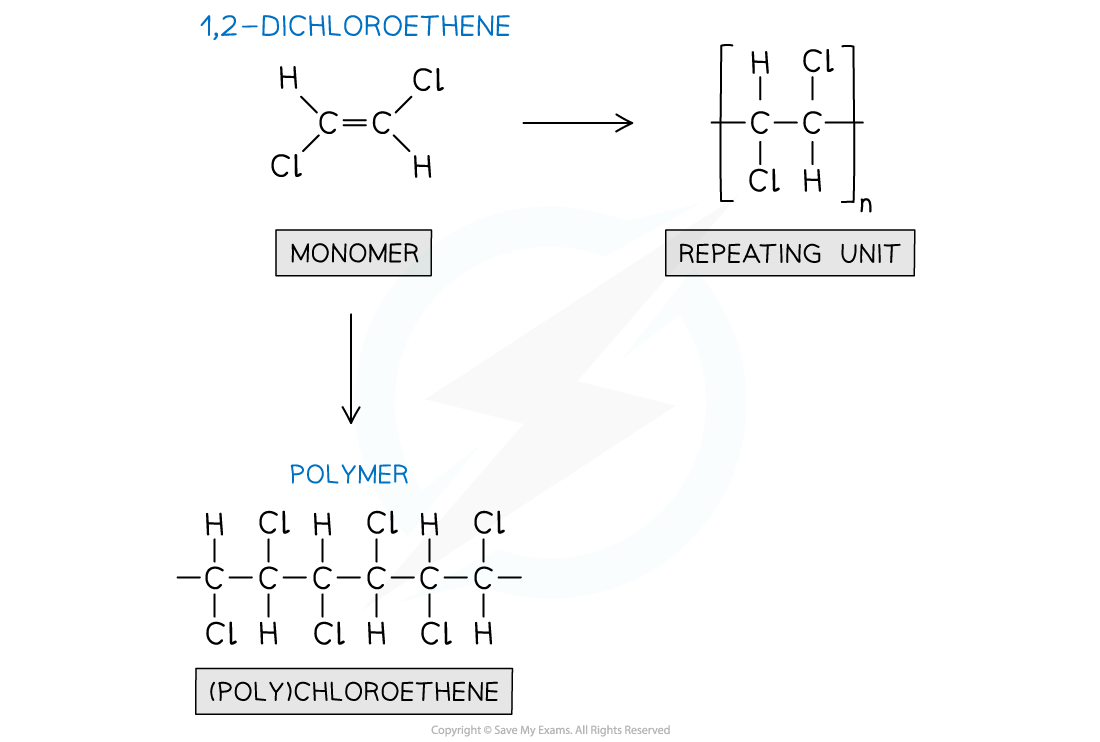
Addition polymerisation of one alkene monomer is polymerised, a (poly)alkene is formed
- (Poly)alkenes can be produced if there are 2 or more alkene monomers as well
- When more than one monomer is used for addition polymerisation, the resulting product is known as a copolymer
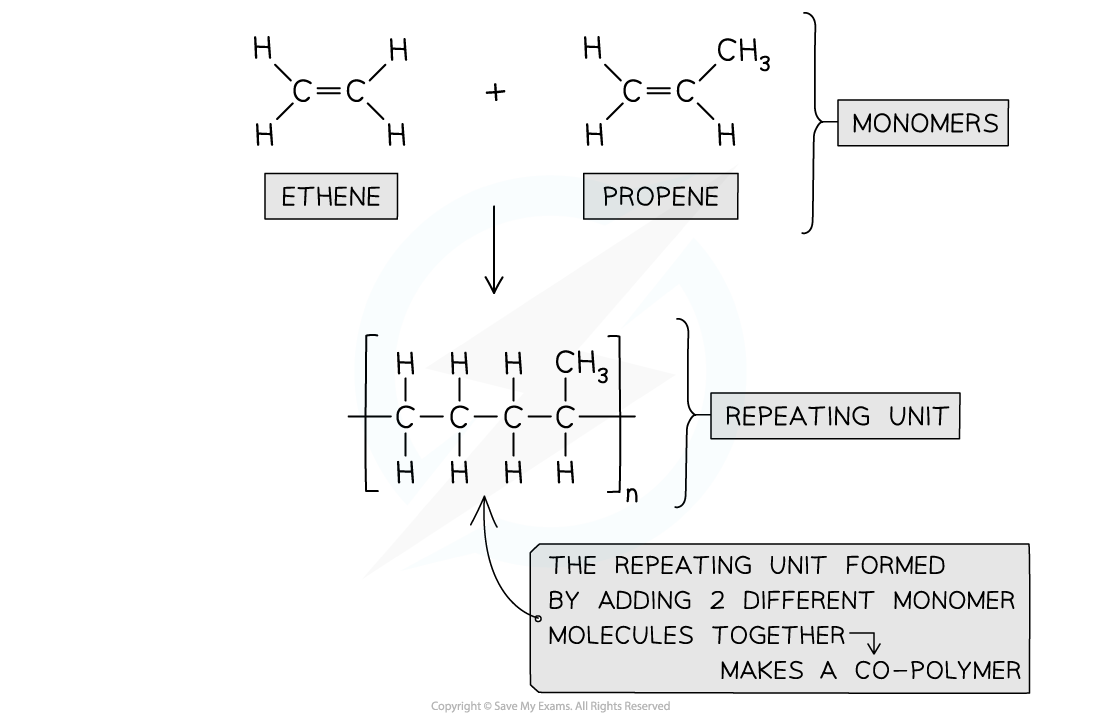 Two or more different alkene monomers can also be polymerised in Addition polymerisation. This gives a co-polymer
Two or more different alkene monomers can also be polymerised in Addition polymerisation. This gives a co-polymer
Condensation polymerisation
- Condensation polymerisation makes polyamides and polyesters
- When looking to identify this type of polymerisation, there are some key functional groups to be aware of
Monomers for condensation polymers table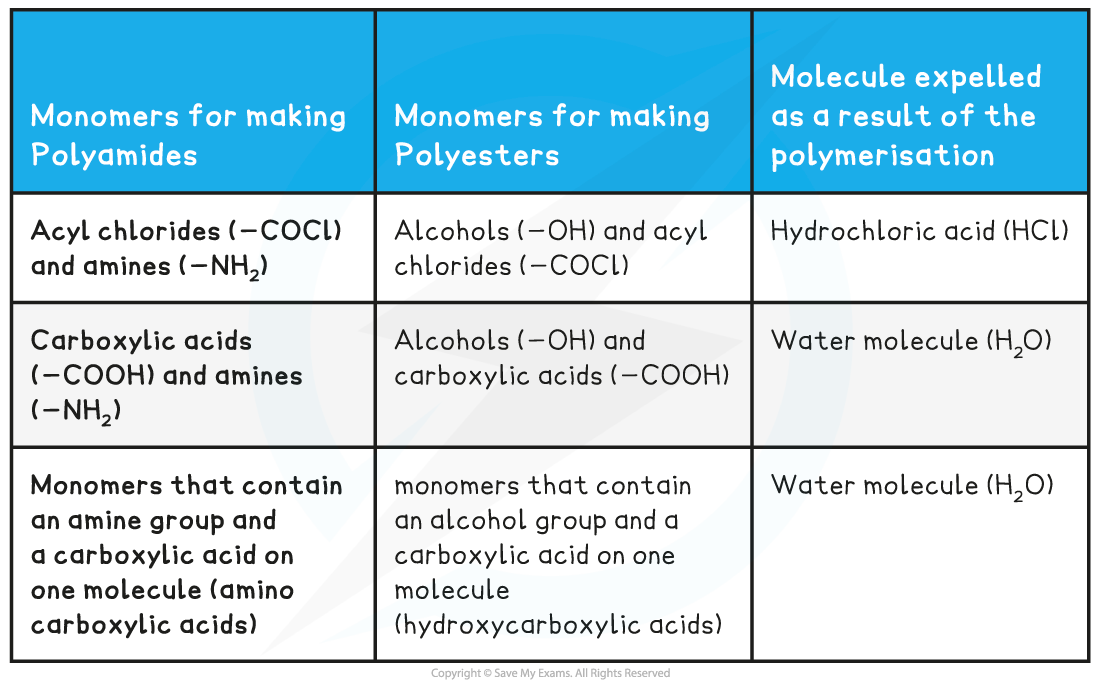
Exam Tip
- As well as the functional groups to be aware of, know that a small molecule is expelled when the polymer is formed
- Identify 2 functional groups that can react together to produce either a polyamide or a polyester
- There are instances where both of the functional groups are on the same monomer molecule
- For example amino acid molecules contain an amine group (-NH2) and a carboxylic acid group (-COOH) therefore it can polymerise to produce a polyamide
Deducing Type of Polymerisation
- The type of polymerisation can be determined by considering the structure of the polymer backbone
Identifying addition polymerisation
- The polymer backbone of an addition polymer does not contain functional groups
- The backbone of the polymer is generally a chain of carbon atoms
- There may be sidechains branching off from the backbone
- Some examples of side chains are benzene rings, nitrile groups (-CN) and halogen atoms (-F/-Cl/-Br/-I)
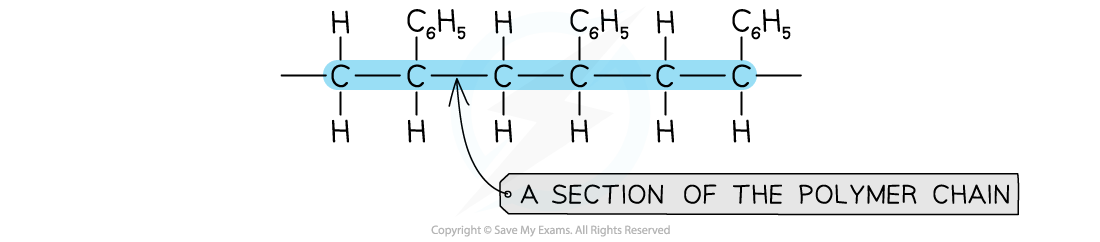
Addition polymers are identified using the plain carbon chain as the polymer backbone
Identifying condensation polymerisation
- A condensation polymer can be identified by functional groups on the polymer backbone
- Polyesters contain ester links and polyamides contain amide/peptide link on the backbone itself
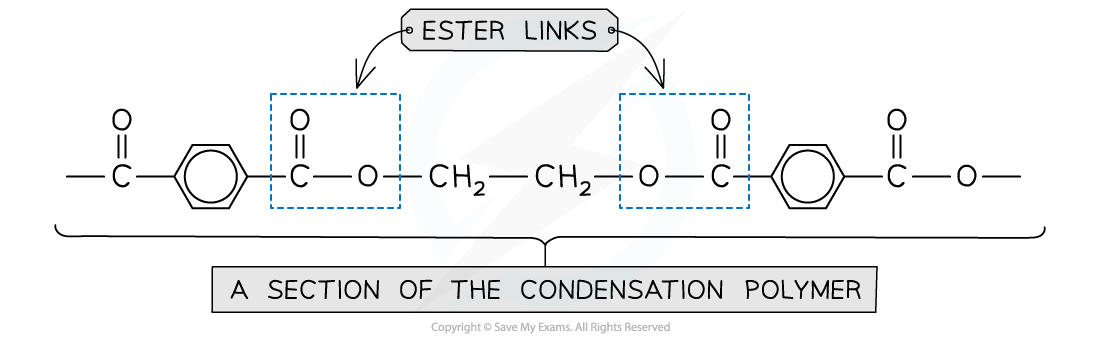
Condensation polymers are identified using function groups that form parts of the polymer backbone
Exam Tip
- Different sections of polymer chains may be formed using various type of polymerisation
- In an exam, you may be given a section of a polymer and asked to determine the type of polymerisation used to form that section
- Firstly, look at the polymer backbone
- If there are functional groups along the backbone, that section was made using condensation polymerisation
- If there are no functional groups along the backbone, addition polymerisation was used
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





