- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记7.7.1 Formation of Polyesters
Formation of Polyesters
- Addition polymerisation has been covered in reactions of alkenes
- They are made using monomers that have C-C double bonds joined together to form polymers such as (poly)ethene
- Condensation polymerisation is another type of reaction and is used in the making of polyesters
- A small molecule (eg. a water molecule) is lost when the monomers join together to form a polyester
- Polyesters contain ester linkages
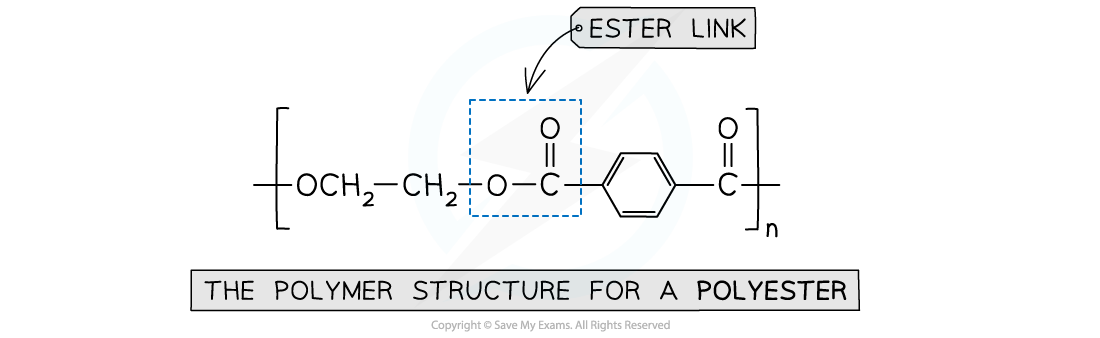
This polymer structure shows an ester functional group linking monomers together
Formation of polyesters
- A diol and a dicarboxylic acid are required to form a polyester
- A diol contains 2 -OH groups
- A dicarboxylic acid contains 2 COOH groups
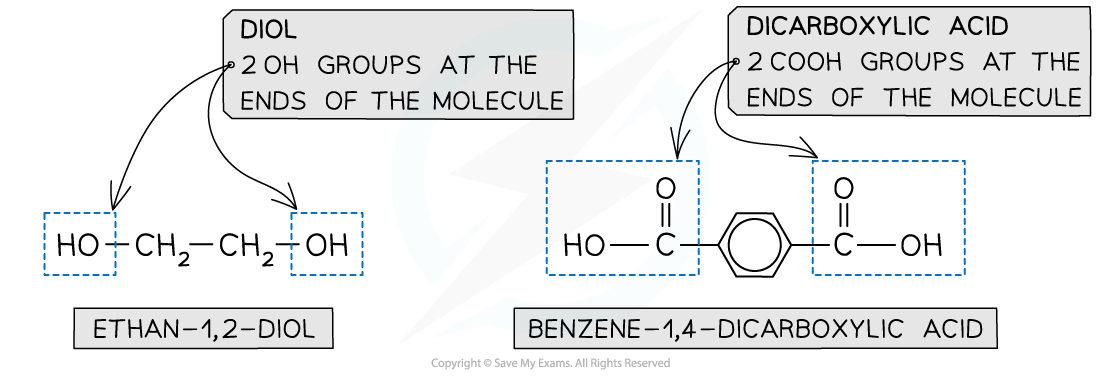
The position of the functional groups on both of these molecules allows condensation polymerisation to take place effectively
- When the polyester is formed, one of the -OH groups on the diol and the hydrogen atom of the -COOH are expelled as a water molecule (H2O)
- The resulting polymer is a polyester
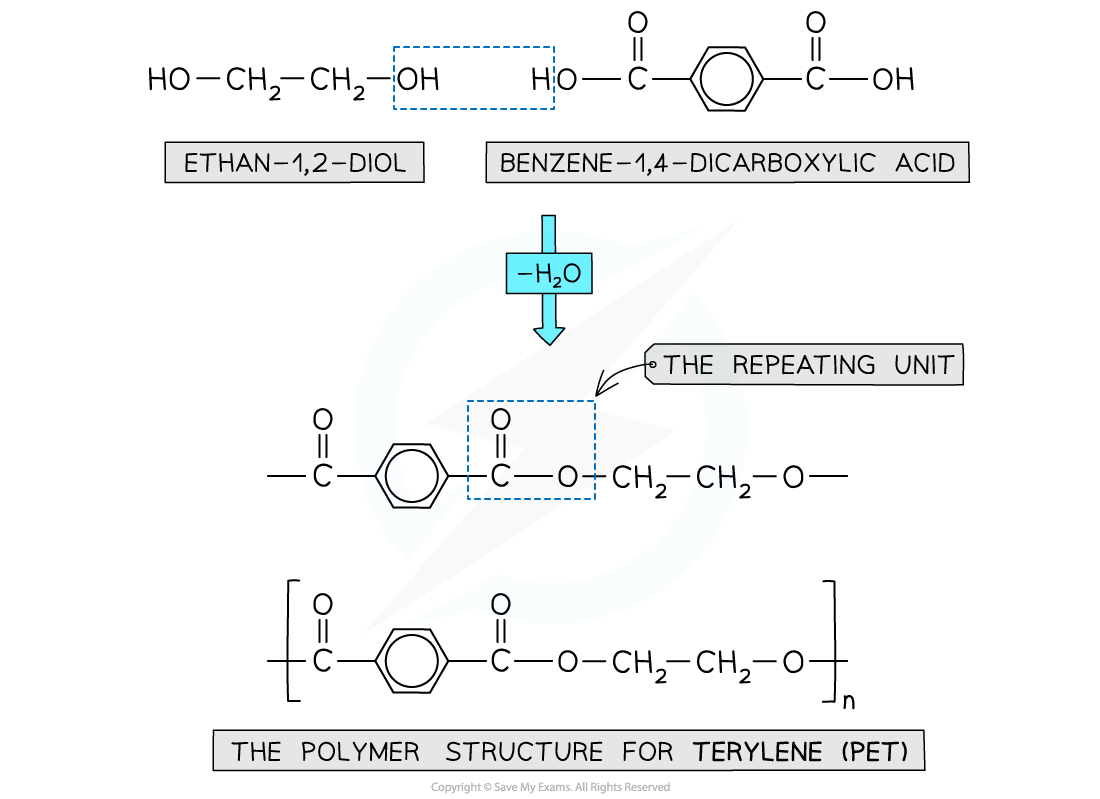 Expulsion of a water molecule in this condensation polymerisation forms the polyester called Terylene (PET)
Expulsion of a water molecule in this condensation polymerisation forms the polyester called Terylene (PET)
Hydroxycarboxylic acids
- So far the examples of making polyesters have focused on using 2 separate monomers for the polymerisation
- There is another route to making polyesters
- A single monomer containing both of the key functional groups can also be used
- These monomers are called hydroxycarboxylic acids
- They contain an alcohol group (-OH) at one end of the molecule while the other end is capped by a carboxylic acid group (-COOH)
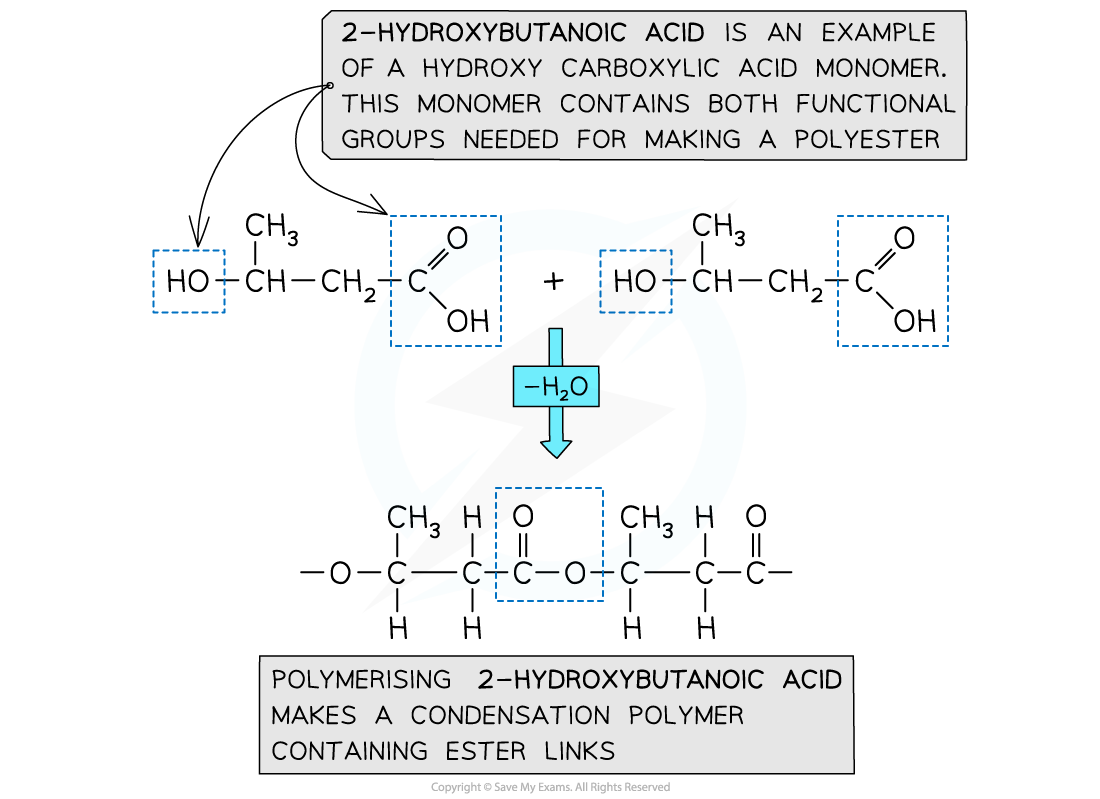
Both functional groups are needed to make a polyester are from the same monomer
Exam Tip
- Polyesters can be made using condensation polymerisation
- The monomers needed are diols and dicarboxylic acids/dioyl chlorides or a single hydroxycarboxylic acid monomer
转载自saveyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





