- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记4.1.5 M + 1 Peak
Determine Number of Carbon Atoms Using M+1 Peak
- The [M+1] peak is caused by the presence of the carbon-13 (13C) isotope in the molecule
- Carbon-13 makes up approximately 1.1% of all carbon atoms
- Therefore, the [M+1] peak is much smaller than the M peak as the isotope is less common
- The ratio of 13C to 12C is approximately 1:99
- Thus, the greater the number of carbon atoms present in a molecule the greater the height of the [M+1] peak
- The number of carbon atoms, n, in a compound can be deduced using the [M+1] peak and the following formula:
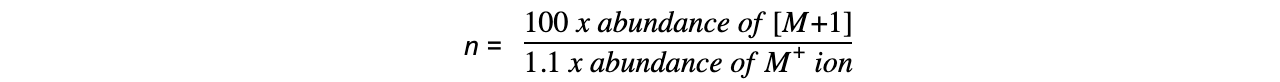
Worked example: Determining number of carbon atoms
Answer
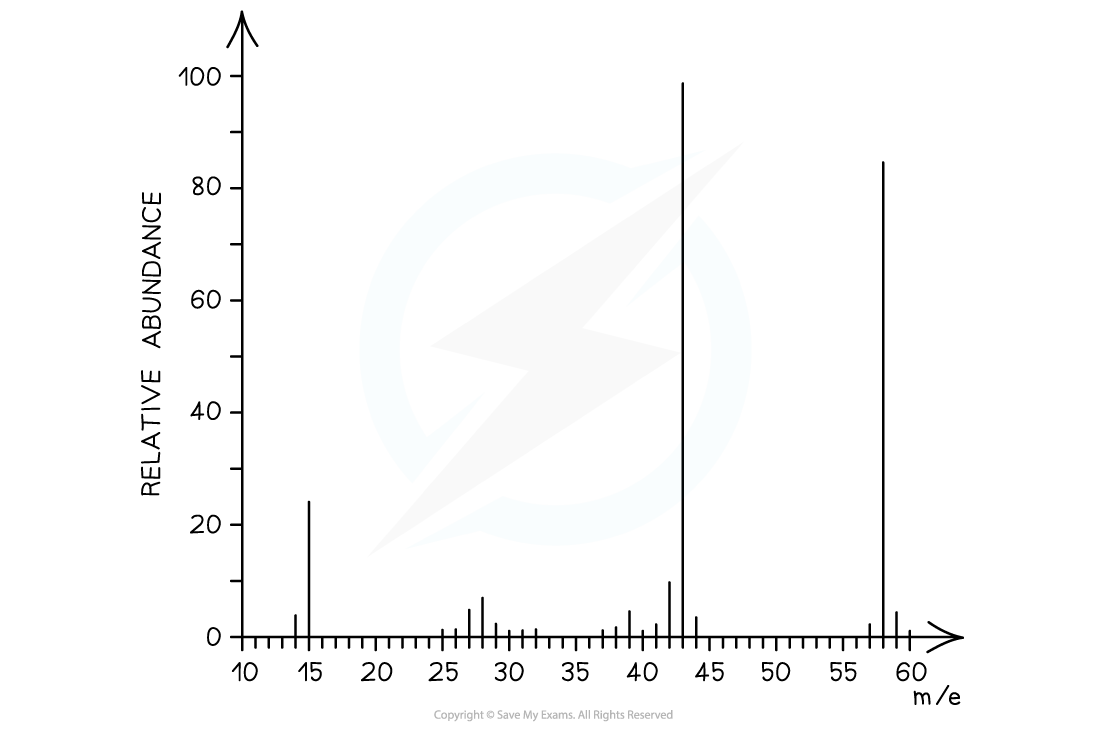
The M+ ion peak is at m/e 58 with a relative abundance of around 85
The [M+1] peak is at m/e 59 with a relative abundance of 3
Therefore, the number of carbon atoms (n) is:
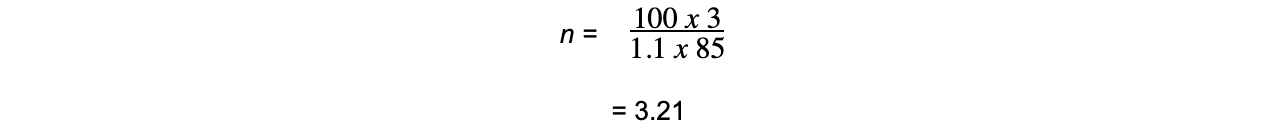
There are therefore 3 carbon atoms present in compound X
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





