- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记4.1.3 Isotopic Abundance & Relative Atomic Mass
Calculating Relative Atomic Mass
- Isotopes are different atoms of the same element that contain the same number of protons and electrons but a different number of neutrons.
- These are atoms of the same elements but with different mass numbers
- Because of this, the mass of an element is given as relative atomic mass (Ar) by using the average mass of the isotopes
- The relative atomic mass of an element can be calculated by using the relative abundance values
- The relative abundance of an isotope is either given or can be read off the mass spectrum
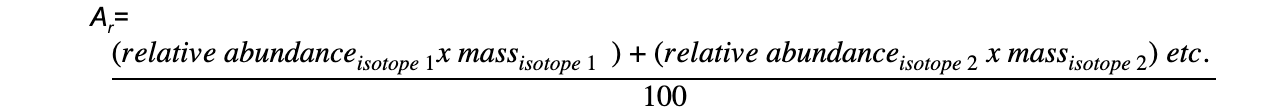
Worked example: Calculating relative atomic mass of oxygen
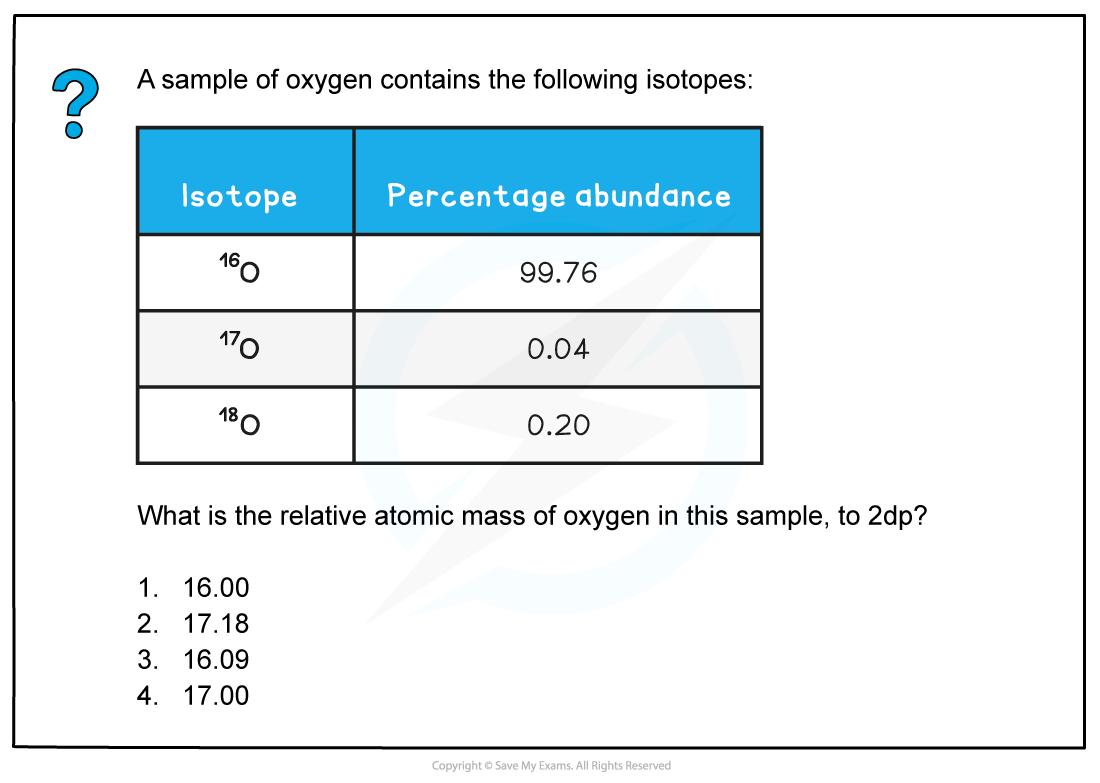
Answer
The correct answer is 1
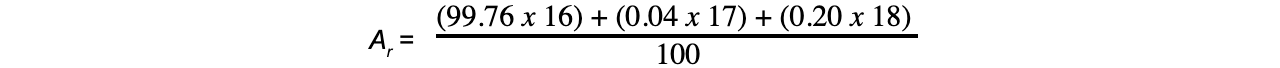
= 16.0044
= 16.00
Worked example: Calculating relative atomic mass of boron

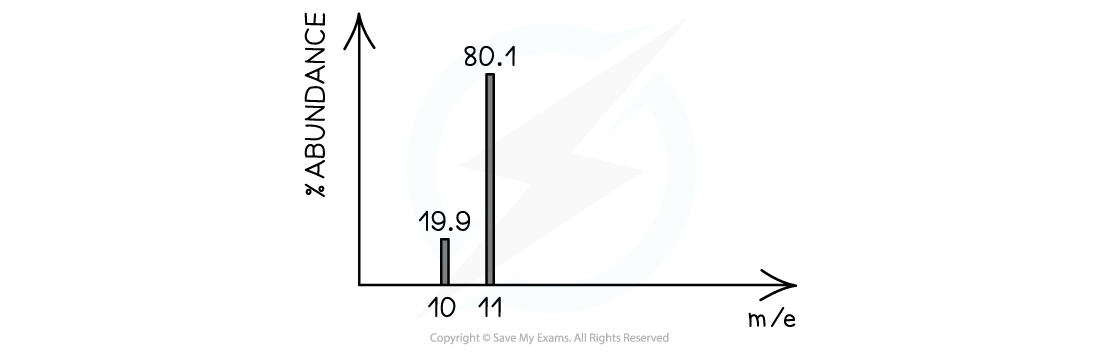
Answer
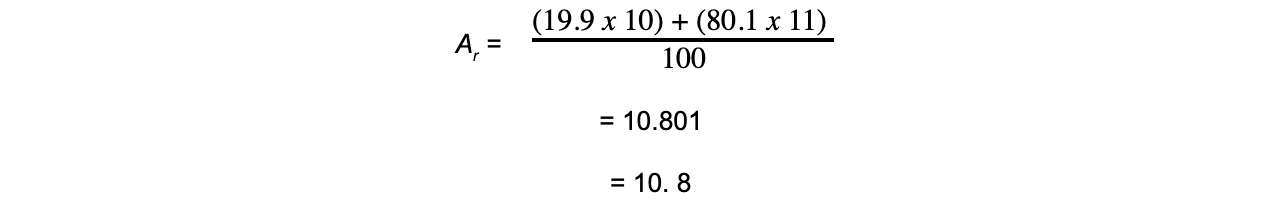
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





