- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记4.1.1 Infra-Red Spectroscopy
Interpreting IR Spectra
- Infrared (IR) spectroscopy is a technique used to identify compounds based on changes in vibrations of atoms when they absorb IR of certain frequencies
- A spectrophotometer irradiates the sample with electromagnetic waves in the infrared region and then detects the intensity of the wavelength of IR radiation which goes through the sample
- All organic molecules absorb IR radiation and depending on which energies of radiation are absorbed, bonds between atoms will vibrate by stretching, bending and twisting
- The molecules will only vibrate at a specific frequency
- The resonance frequency is the specific frequency at which the molecules will vibrate to stimular larger vibrations
- Depending on the rest of the molecule, each vibration will absorb specific wavelengths of IR radiation which are also shown as the reciprocal of the wavelength
- This unit is called the wavenumber (cm-1)
- Particular absorbance have characteristic widths (broad or sharp) and intensities (strong or weak)
- For example, hydrogen bonds cause the O-H bonds in alcohols and carboxylic acids to be broad whereas the C-O bond in carbonyl (C=O) groups have a strong, sharp absorbance peak
- The energies absorbed by different functional groups are given as a range and an unknown compound can be identified by comparing its IR spectrum to the IR spectrum of a known compound
Absorption range of bonds table
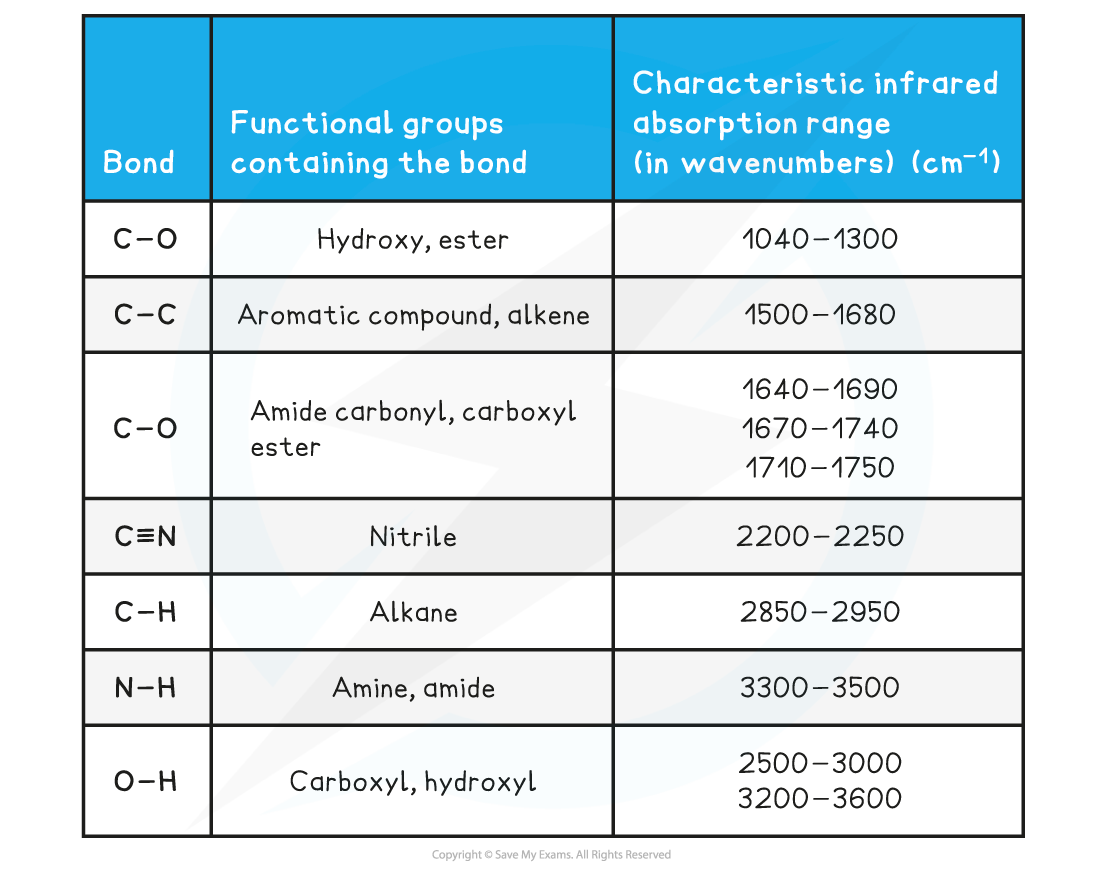
- Due to some absorption bands overlapping each other, other analytical techniques such as mass spectroscopy should be used alongside IR spectroscopy to identify an unknown compound
Worked Example: Analysing IR spectra

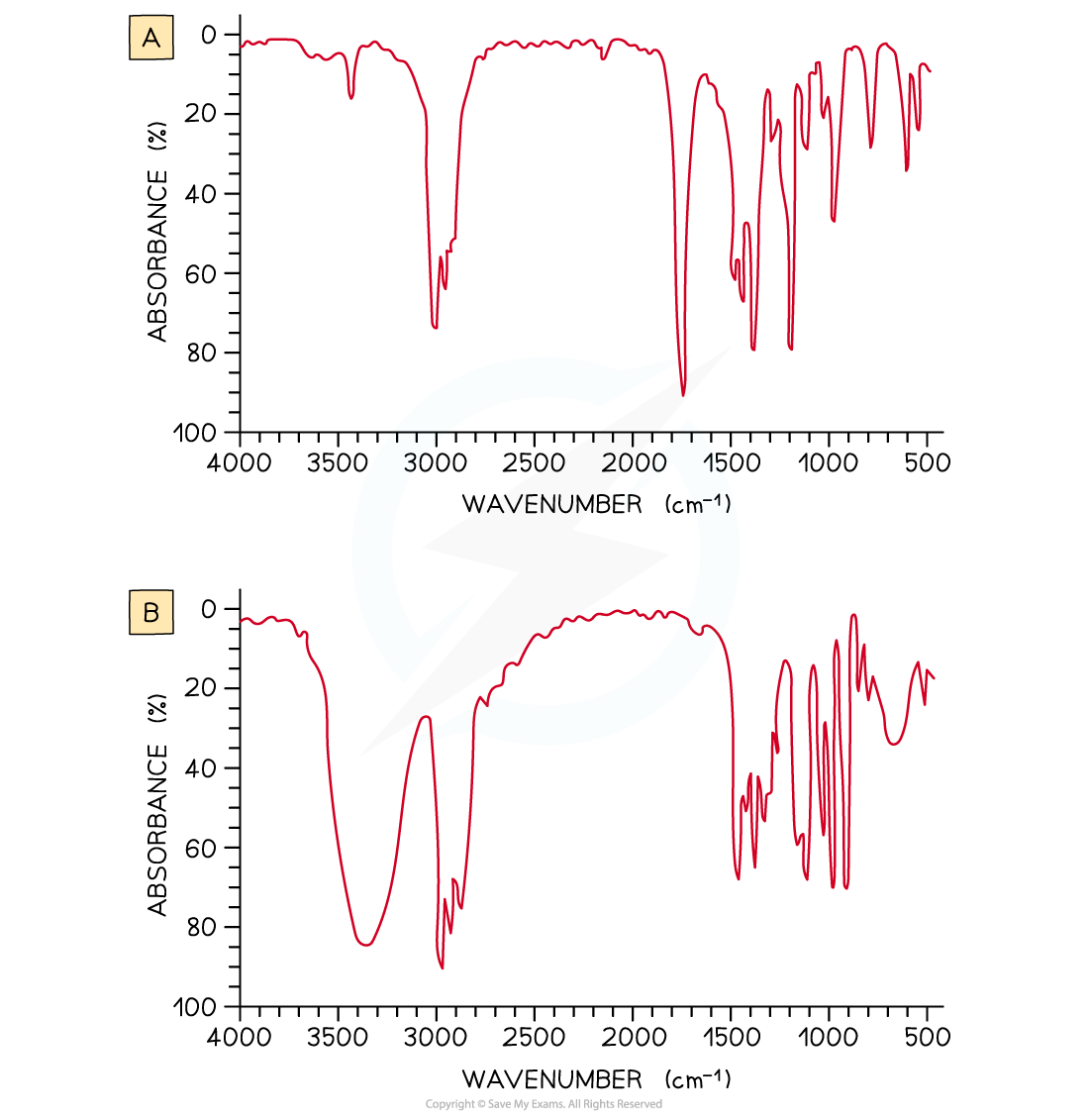
Answer
IR spectrum A is propanone and spectrum B is propan-2-ol.
In IR spectrum A the presence of a strong, sharp absorption around 1710 cm-1 corresponds to the characteristic C=O, carbonyl, group in a ketone.
In spectrum B the presence of a strong, broad absorption around 3200-3500 cm-1 suggests that there is an alcohol group present, which corresponds to the -OH group in propan-2-ol.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





