- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.6.2 Redox Reactions
Balancing Redox Reactions
- Oxidation numbers can be used to balance chemical equations
- Roman numerals between brackets are used to show the ox. no. of an atom that can have multiple oxidation states, eg:
Fe(III) = iron with ox. no. +3
Worked example: Writing overall redox reactions
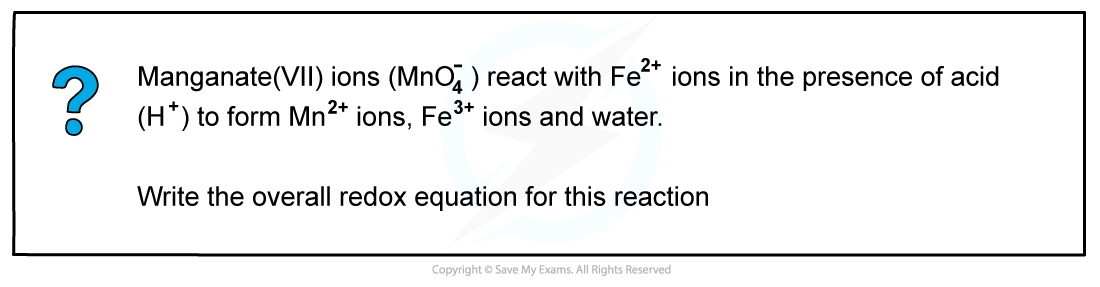
Answer
- Step 1: Write the unbalanced equation and identify the atoms which change in ox. no.

- Step 2: Deduce the ox.no. changes
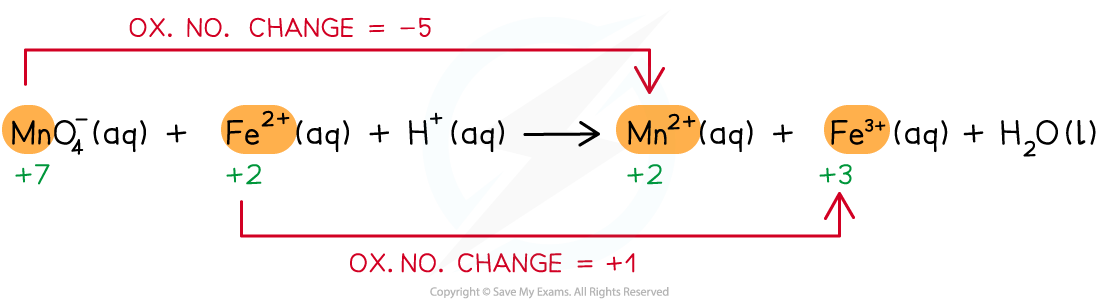
- Step 3: Balance the ox.no. changes
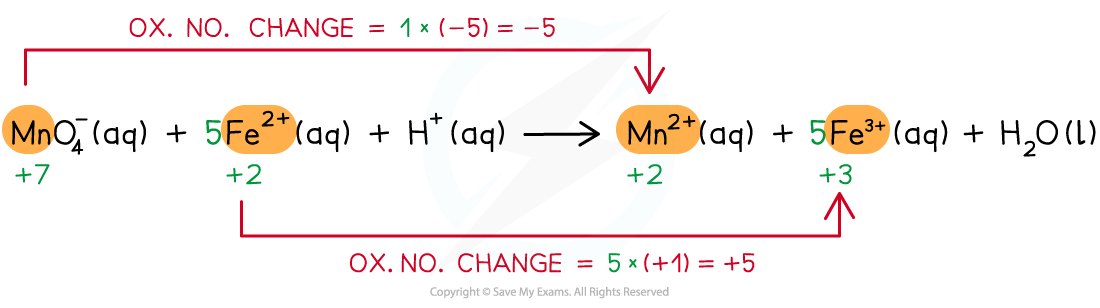
- Step 4: Balance the charges
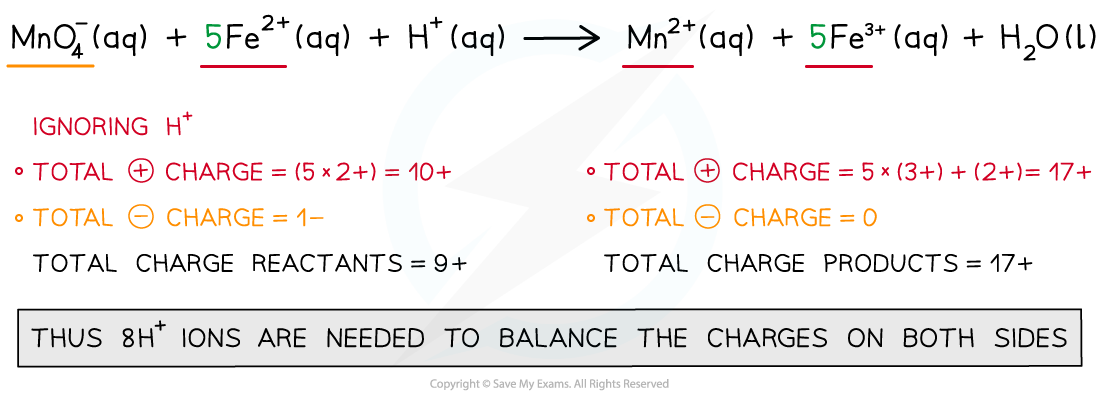
- Step 5: Balance the atoms

Redox & Disproportionation Reactions
Oxidation
- Oxidation is the gain of oxygen, eg:
Cu + H2O → CuO + H2
(Cu has gained an oxygen and is oxidised)
- Oxidation is also the loss of a hydrogen, eg:
2NH3 + 3Br2 → N2 + 6HBr
(N has lost a hydrogen and is oxidised)
- Oxidation is also the loss of electrons, eg:
Cu2+ → Mg → Mg2+ + Cu
(Mg has lost two electrons and is oxidised)
- Oxidation causes an increase in ox. no., eg:
Cu2+ + Mg → Mg2+ + Cu
(change in ox. no. of Mg is +2 thus Mg is oxidised)
Reduction
- Reduction is the loss of oxygen, eg:
Cu+ H2O → 2CuO + H2
(O has been reduced)
- Reduction is also the gain of a hydrogen, eg:
2NH3+ 3Br2 → N2 + 6HBr
(Br has been reduced)
- Reduction is also the gain of electrons, eg:
Cu2+ + Mg → Mg2+ + Cu
(Cu has been reduced)
- Reduction causes a decrease in oxidation number, eg:
Cu2+ + Mg → Mg2+ + Cu
(change in ox. no. of Cu is -2 thus Cu is reduced)
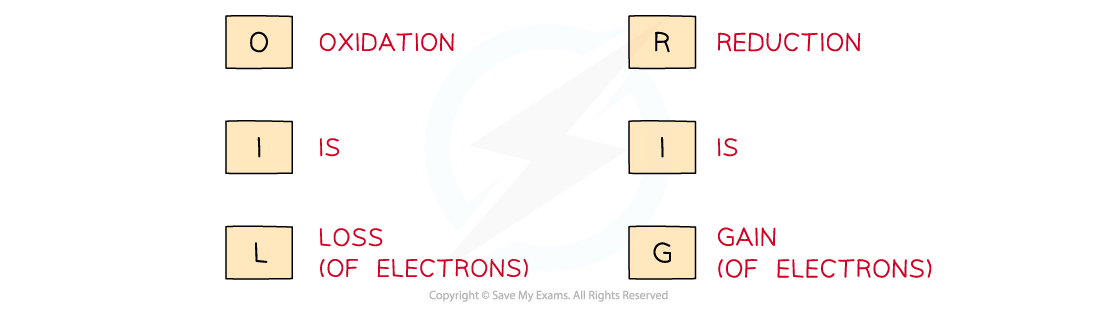
Use the acronym "Oil Rig" to help you remember the definitions of oxidation and reduction
Redox reactions
- Redox reactions are reactions in which oxidation and reduction take place together
- While one species is oxidising, another is reducing in the same reaction, eg:
Cu2++ Mg → Mg2+ + Cu
(Cu has been reduced and Mg has been oxidised)
Worked example: Oxidation and reduction
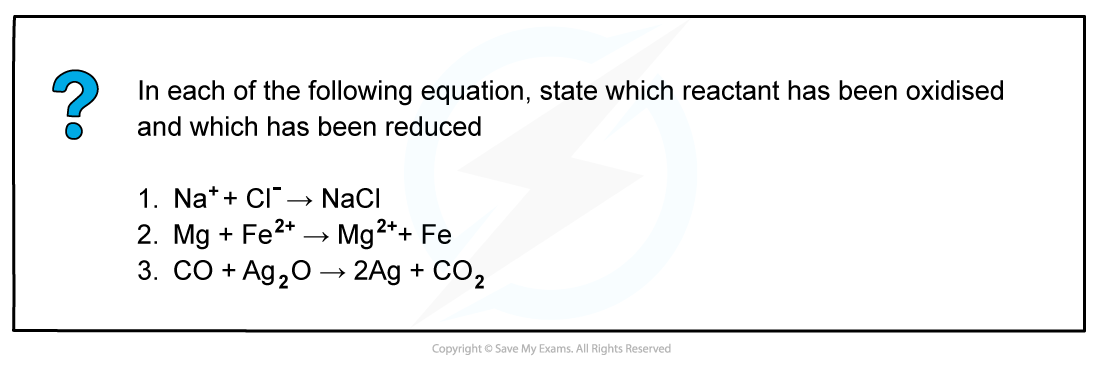
Answer
Answer 1:
Oxidised: Cl- as the ox. no. has increased by 1Reduced: Na+ as the ox. no. has decreased by 1
Answer 2:
Oxidised: Mg as the ox. no. has increased by 2
Reduced: Fe2+ as the ox. no. has decreased by 2
Answer 3:
Oxidised: C as it has gained oxygenReduced: Ag as it has lost oxygen
Disproportionation reactions
- A disproportionation reaction is a reaction in which the same species is both oxidised and reduced
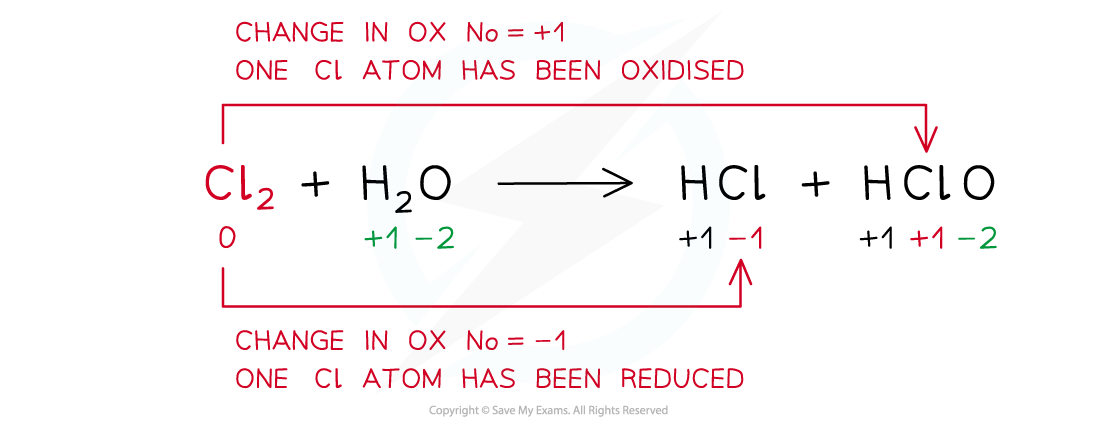 Example of a disproportion reaction in which the same species (chlorine in this case) has been both oxidised and reduced
Example of a disproportion reaction in which the same species (chlorine in this case) has been both oxidised and reduced
Worked example: Balancing disproportionation reactions

Answer
- Step 1: Write the unbalanced equation and identify the atoms that change in ox. no.

- Step 2: Deduce the ox. no. changes
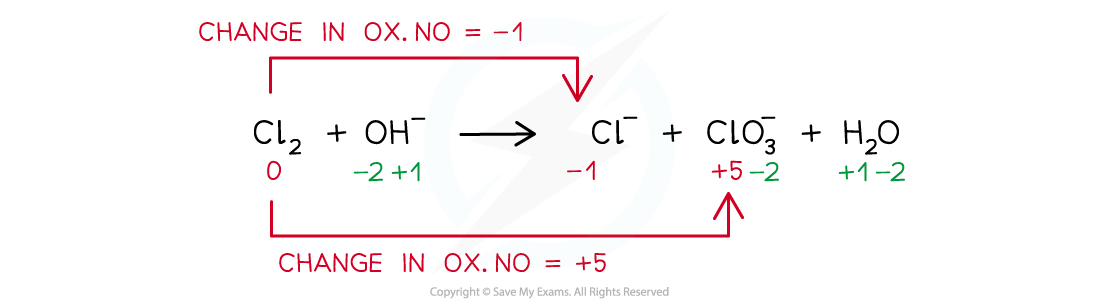
- Step 3: Balance the ox. no. changes
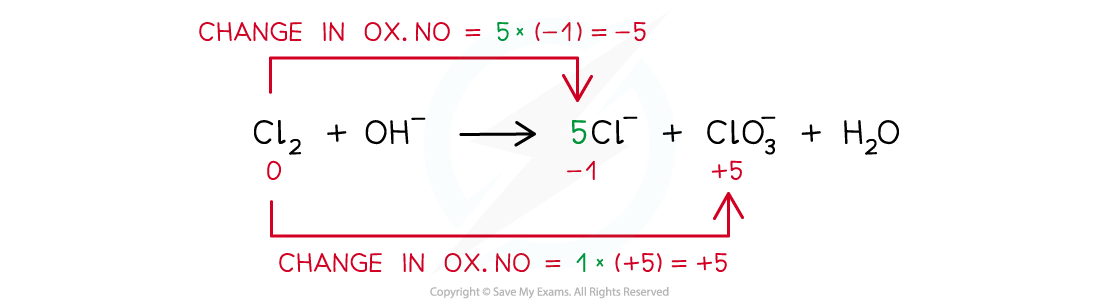
- Step 4: Balance the charges

- Step 5: Balance the atoms

转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





