- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.6.1 Oxidation Number Rules
Oxidation Numbers
- The oxidation numbers (also known as oxidation state) is a number given to each atom or ion in a compound to keep track of how many electrons they have
- In a single ion or molecular ion, the oxidation number tells us how many electrons have been lost or gained
- Positive oxidation number = loss of electrons
- Negative oxidation number = gain of electrons
Oxidation number rules
- The oxidation number (ox.no.) refers to a single atom in a compound

Worked example: Deducing oxidation numbers
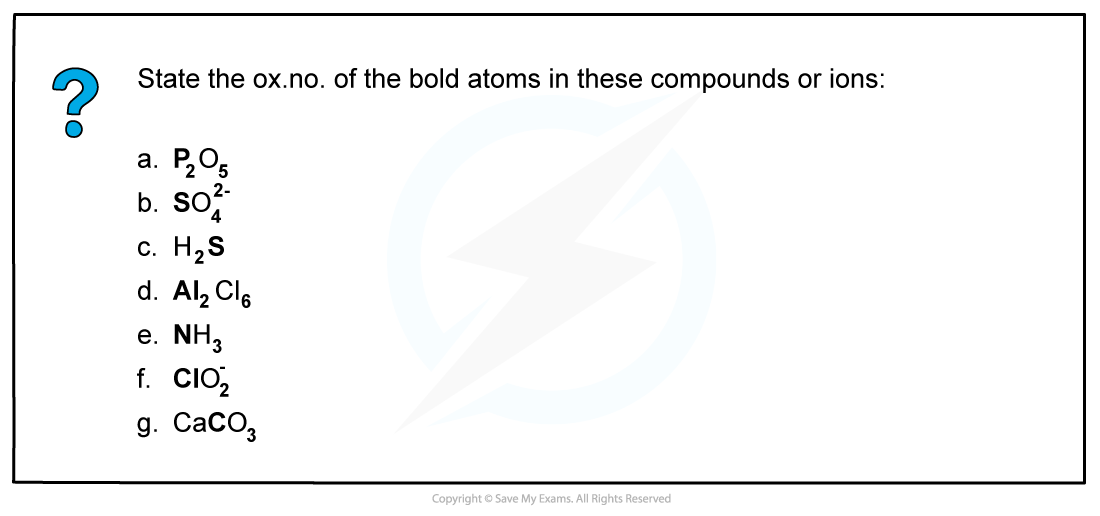
Answer

转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





