- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.5 Ionic Bonding Examples
Ionic Bonding: Examples
Sodium chloride
- Sodium is a Group 1 metal
- It loses its outer electron to form a sodium ion with a +1 charge (Na+)
- Chlorine is a Group 7 non-metal
- It gains 1 electron to form a chloride ion with a -1 charge (Cl-)
- The oppositely charged ions are attracted to each other by electrostatic forces to form NaCl (ionic bonds)
- The final ionic solid is neutral in charge
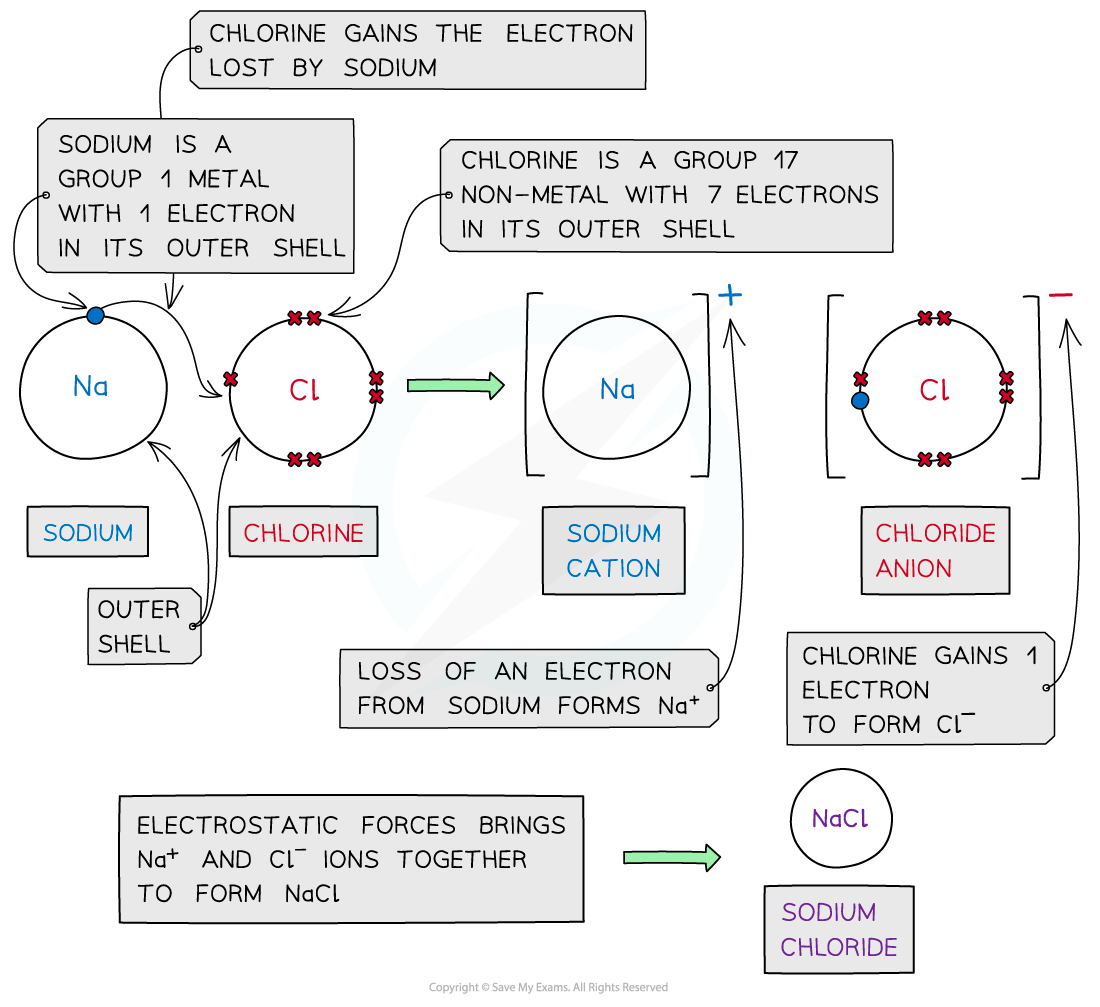
Dot and cross diagram to show the ionic bonding in sodium chloride
Magnesium oxide
- Magnesium is a Group 2 metal
- It loses its 2 outer electrons to form a magnesium ion with a +2 charge (Mg2+)
- Oxygen is a Group 6 non-metal
- It gains 2 electrons to form an oxide ion with a -2 charge (O2-)
- The oppositely charged ions are attracted to each other to by electrostatic forces to form MgO (ionic bonds)
- The final ionic solid is neutral in charge
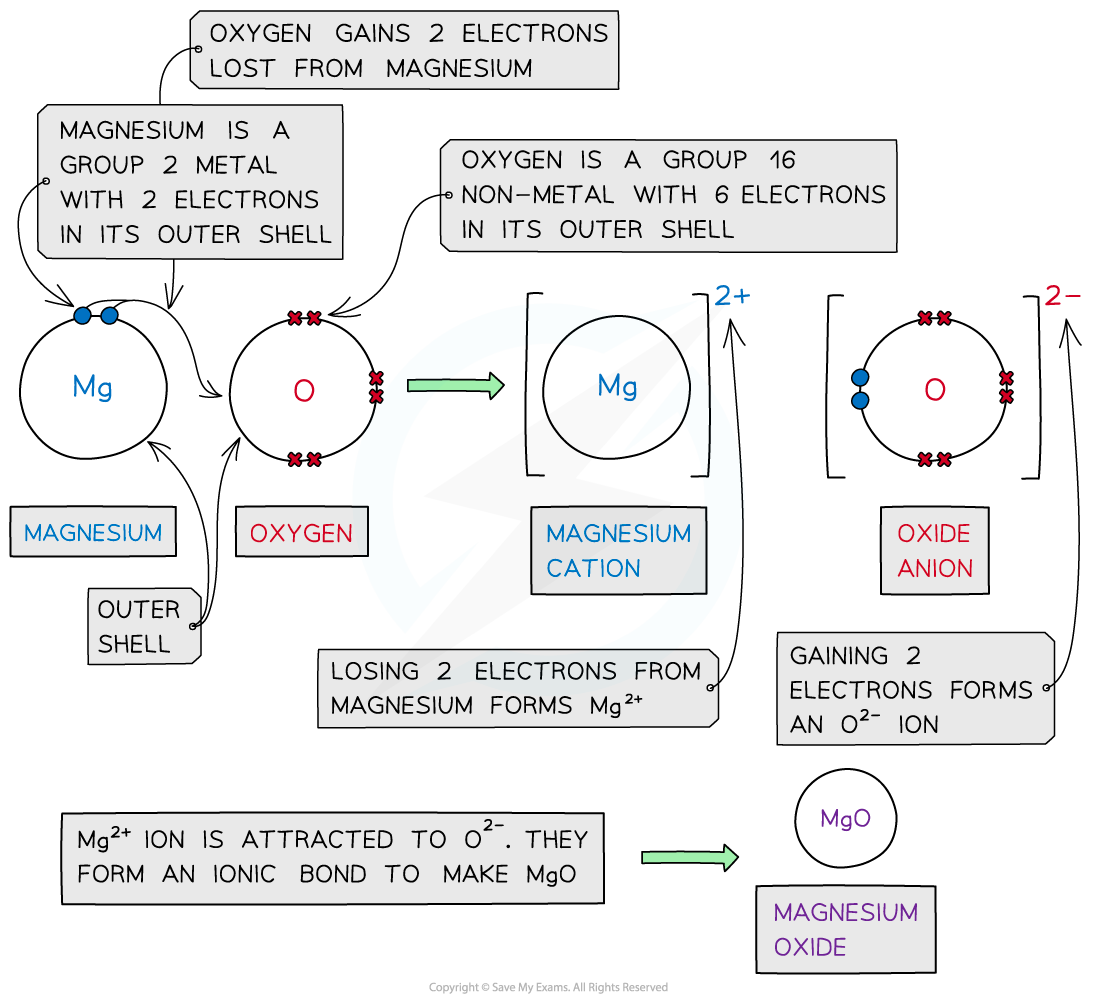
Dot and cross diagram to show the ionic bonding in magnesium oxide
Calcium fluoride
- Calcium is a Group 2 metal
- It loses its 2 outer electrons to form a calcium ion with a +2 charge (Ca2+)
- Fluorine is a Group 7 non-metal
- It gains 1 electron to form a fluoride ion with a -1 charge (F-)
- As before, the positive and negative ions are attracted to each other via an ionic bond
- However, to cancel out the 2+ charge of the calcium ion, 2 fluorine atoms are needed
- Each fluorine atom can only accept 1 electron from the calcium atom
- 2 fluoride ions will be formed
- Calcium fluoride is made when 1 calcium ion and 2 fluoride ions form ionic bonds, CaF2
- The final ionic solid of CaF2 is neutral in charge
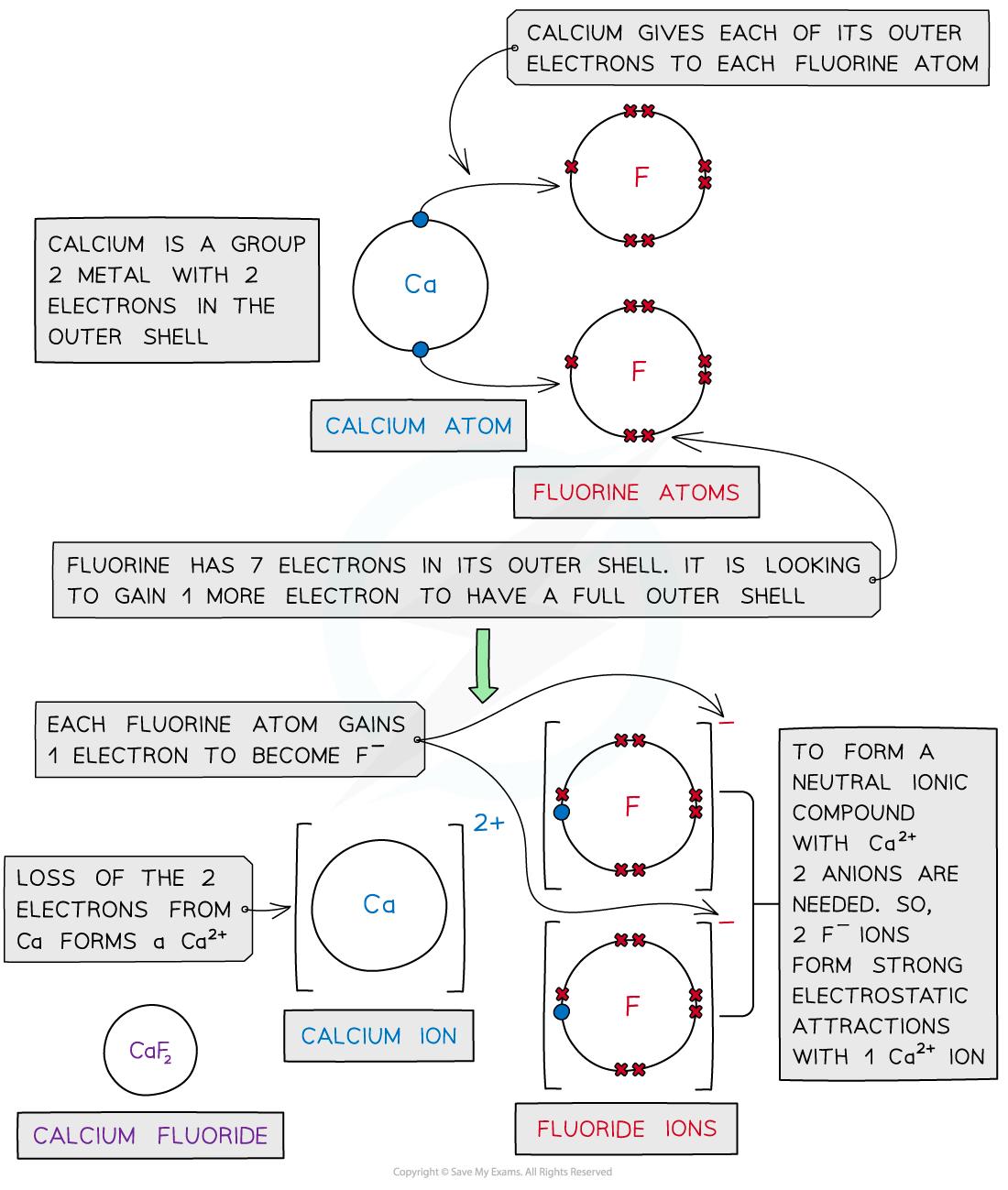
Dot and cross diagram to show the ionic bonding in calcium fluoride
Worked example: Dot & cross lithium nitride

Answer
- Lithium is a Group 1 metal
- It loses its outer electron to form a lithium ion with a +1 charge (Li+)
- Nitrogen is a Group 5 non-metal
- It gains 3 electrons to form a nitride ion with a -3 charge (N3-)
- To cancel out the -3 charge of the nitride ion, 3 lithium atoms are needed and 3 lithium ions will be formed
- Lithium nitride is made when 1 nitride ion and 3 lithium ions form ionic bonds
- The final ionic solid of Li3N is neutral in charge
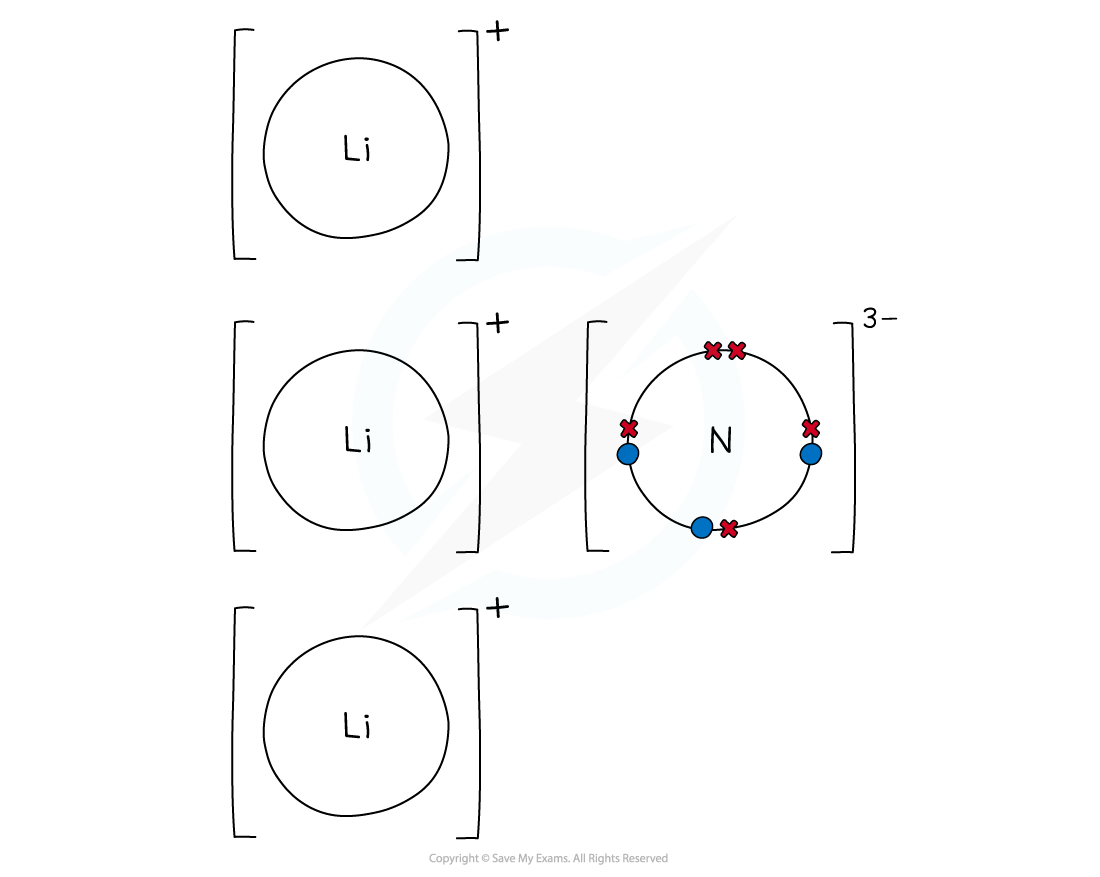
Dot and cross diagram to show the ionic bonding in lithium nitride
Worked example: Dot & cross aluminium oxide

Answer
- Aluminium is a Group 3 metal
- It loses its outer electrons to form an aluminium ion with a +3 charge (Al3+)
- Oxygen is a Group 6 non-metal
- It gains 2 electrons to form an oxide ion with a -2 charge (O2-)
- To cancel out the negative and positive charges, 2 aluminium and 3 oxygen atoms are needed
- Aluminium oxide is made when 2 aluminium ions and 3 oxygen ions form ionic bonds
- The final ionic solid of Al2O3 is neutral in charge
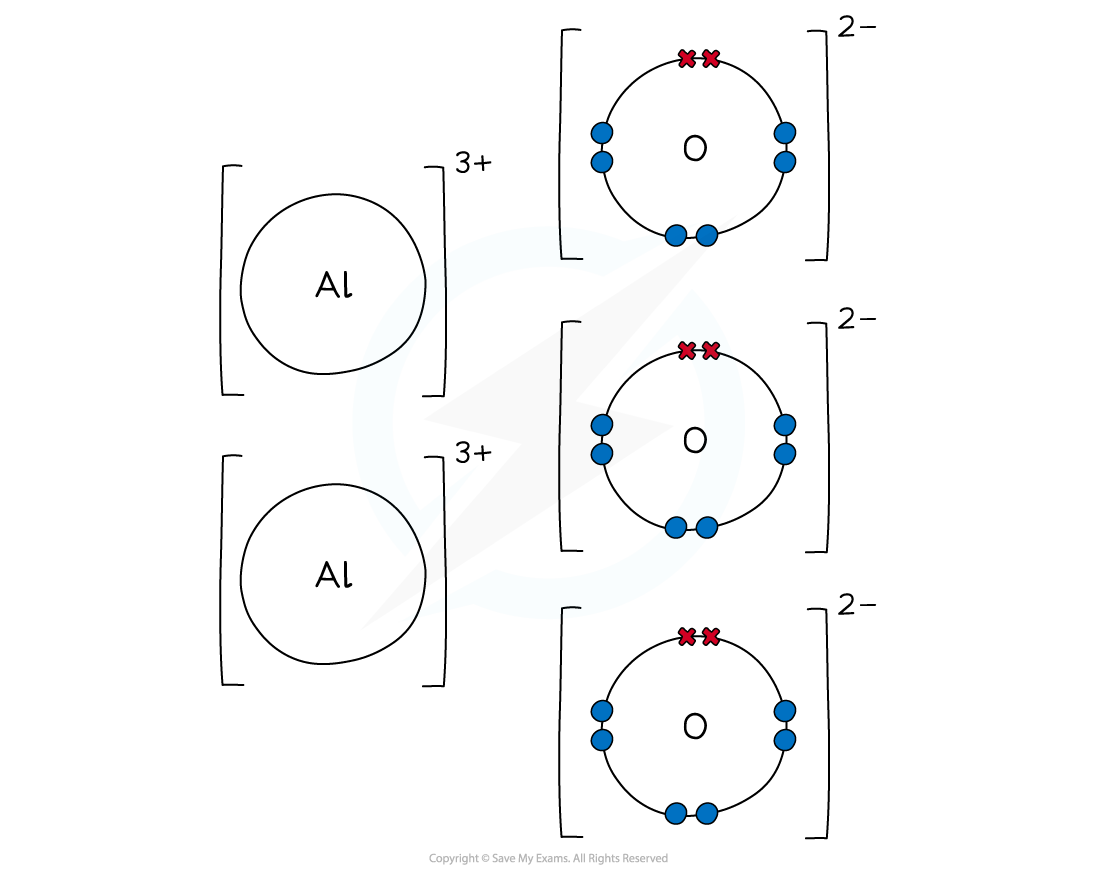
Dot and cross diagram to show the ionic bonding in aluminium oxide
转载自savemexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





