- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.3 Electronegativity & Bonding
Electronegativity: Predicting Bond Formation
- The differences in Pauling electronegativity values can be used to predict whether a bond is covalent or ionic in character
Electronegativity & covalent bonds
- Single covalent bonds are formed by sharing a pair of electrons between two atoms
- In diatomic molecules the electron density is shared equally between the two atoms
- Eg. H2, O2 and Cl2
- Both atoms will have the same electronegativity value and have an equal attraction for the bonding pair of electrons leading to formation of a covalent bond
- The equal distribution leads to a non-polar molecule
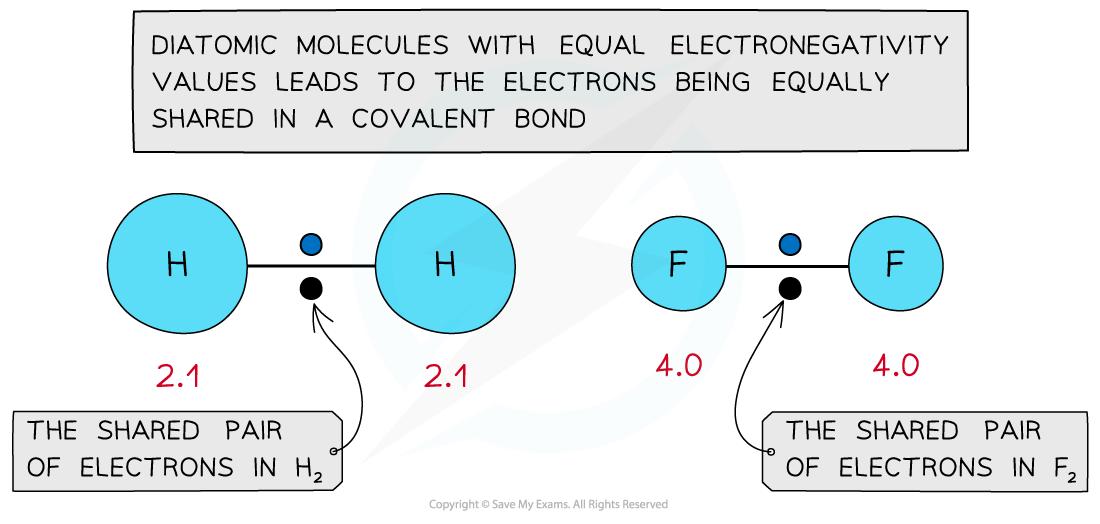
The electronegativity values are equal resulting in the formation of a nonpolar covalent bond
Electronegativity & ionic bonds
- When atoms of different electronegativities form a molecule, the shared electrons are not equally distributed in the bond
- The more electronegative atom (the atom with the higher value on the Pauling scale) will draw the bonding pair of electrons towards itself
- A molecule with partial charges forms as a result
- The more electronegative atom will have a partial negative charge (delta negative, δ-)
- The less electronegative atom will have a partial positive charge (delta positive, δ+)
- This leads to a polar covalent molecule
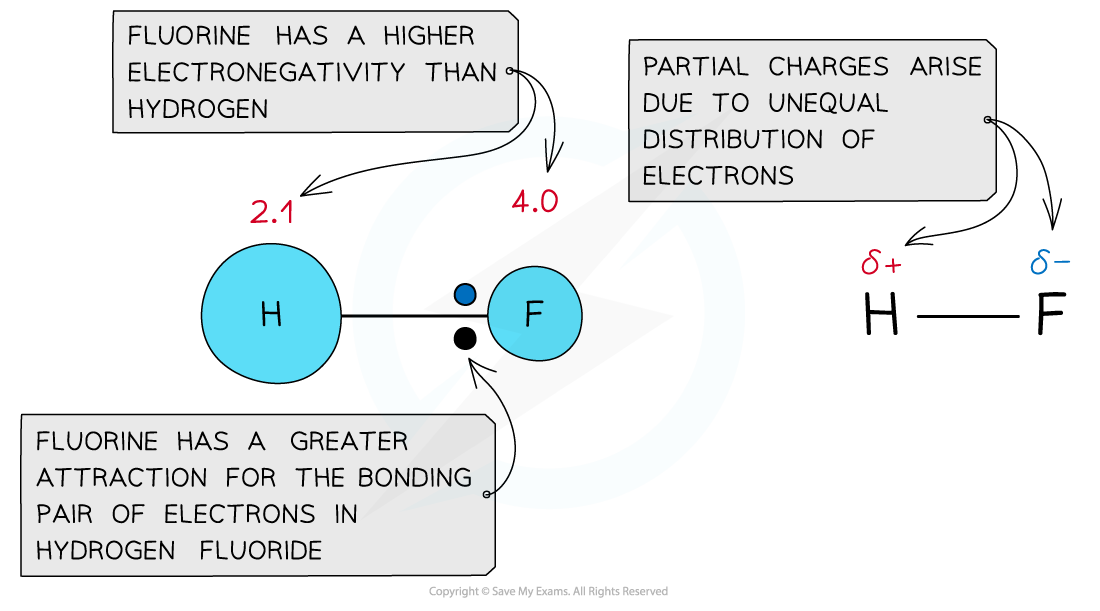
The electronegativity values are not equal - polar bond forms
- If there is a large difference in electronegativity of the two atoms in a molecule, the least electronegative atom’s electron will transfer to the other atom
- This in turn leads to an ionic bond – one atom transfers its electron and the other gains that electron
- The cation is a positively charged species which has lost (an) electron(s)
- The anion is a negatively charged species which has gained (an) electron(s)
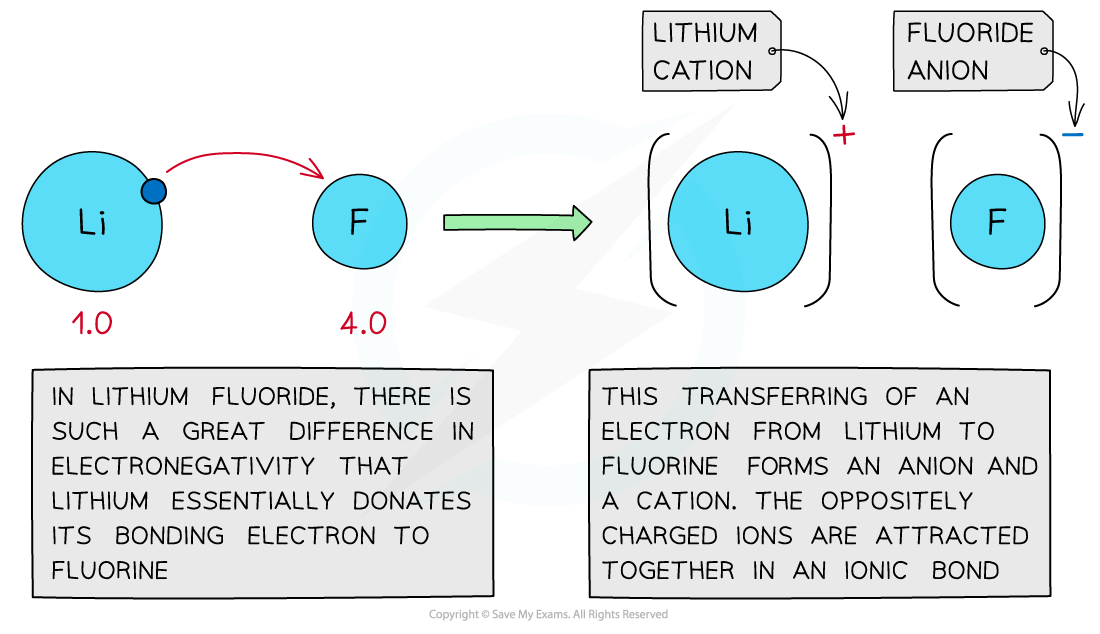
Large differences in electronegativity values lead to the formation of ionic bonds
Exam Tip
You can use the Pauling scale to decide whether a bond is polar or nonpolar: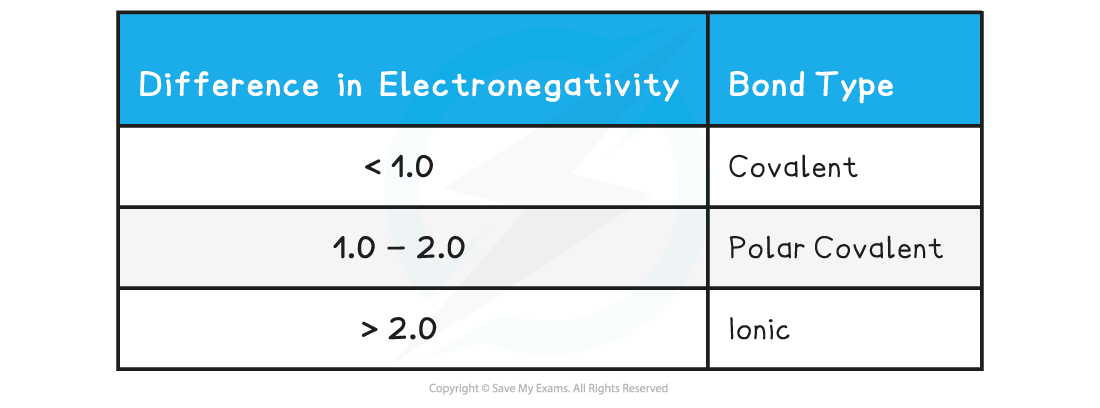
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





