- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.2 Electronegativity Trends
Electronegativity: Trends
- Electronegativity varies across Periods and down the Groups of the Periodic Table
Down a group
- There is a decrease in electronegativity going down the Group
- The nuclear charge increases as more protons are being added to the nucleus
- However, each element has an extra filled electron shell, which increases shielding
- The addition of the extra shells increases the distance between the nucleus and the outer electrons resulting in larger atomic radii
- Overall, there is decrease in attraction between the nucleus and outer bonding electrons
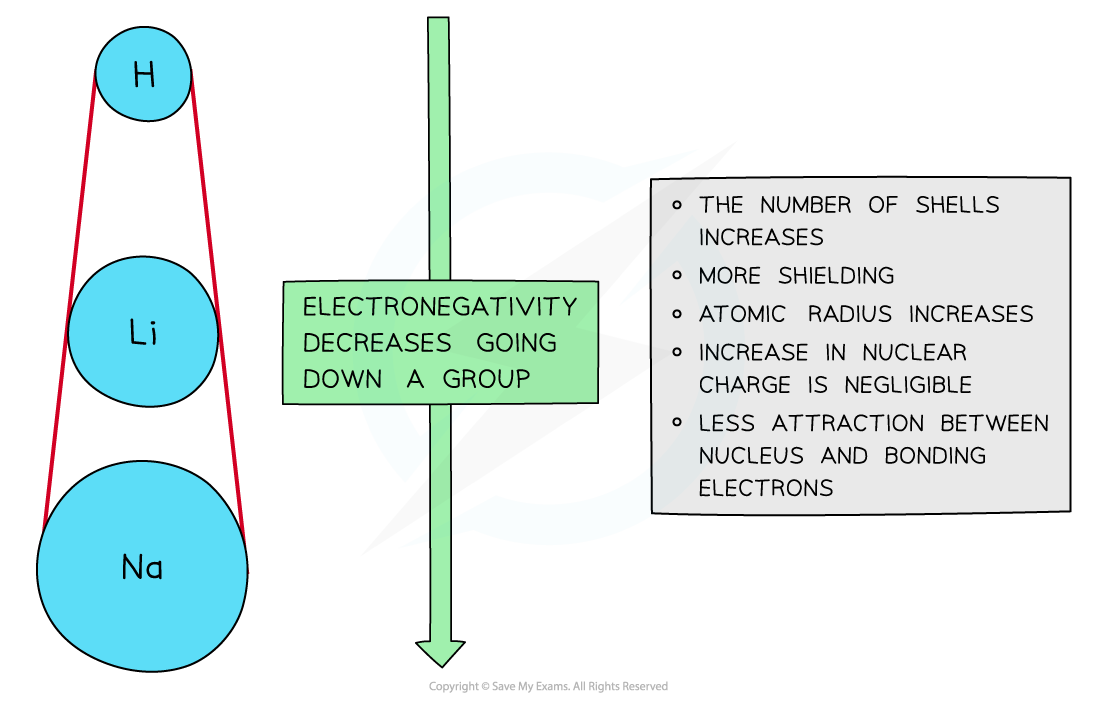
Electronegativity decreases going down the groups of the periodic table
Across a period
- Electronegativity increases across a Period
- The nuclear charge increases with the addition of protons to the nucleus
- Shielding remains reasonably the same across the Period as no new shells are being added to the atoms
- The nucleus has an increasingly strong attraction for the bonding pair of electrons of atoms across the Period of the Periodic Table
- This results in smaller atomic radii
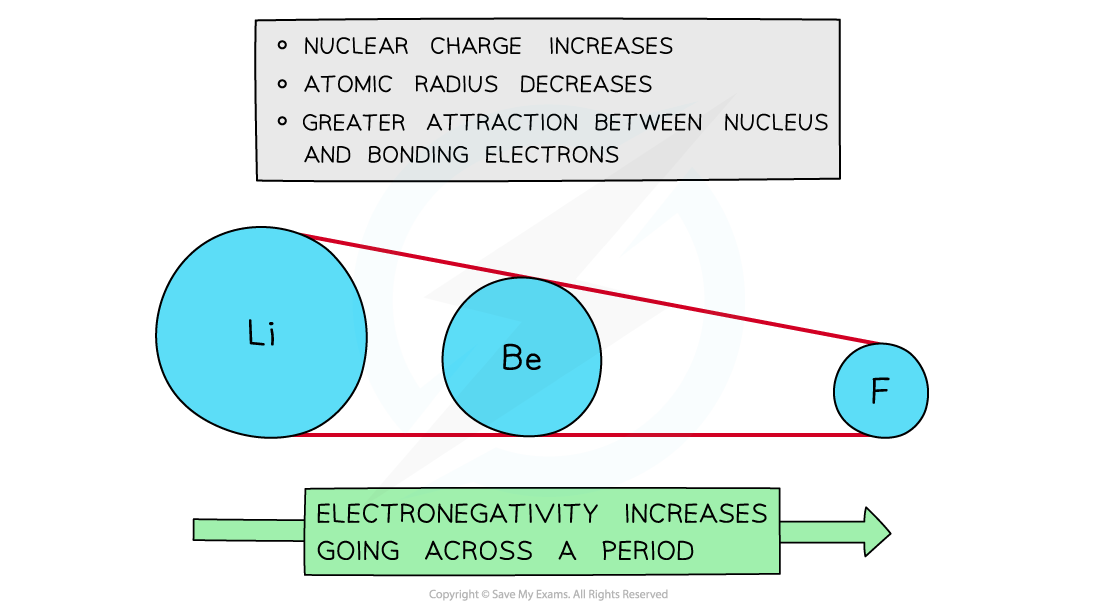
Electronegativity increases going across the periods of the Periodic Table
Trends down a group & across a period table
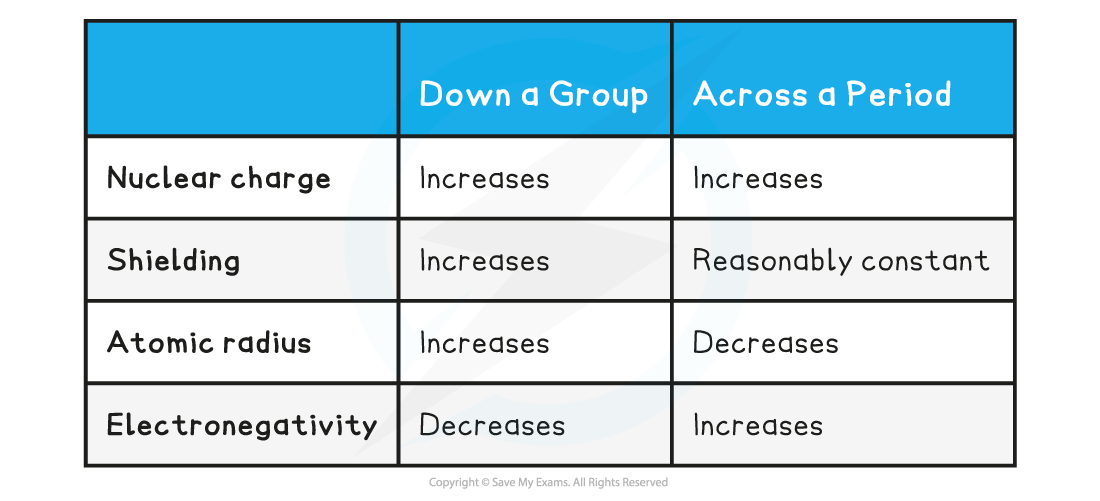
Exam Tip
Remember the general trend is an increase in electronegativity towards the top right of the Periodic Table.Fluorine is the most electronegative element in the periodic table.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





