- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: SL复习笔记3.1.2 Temperature
Temperature
- Temperature is a measure of how hot or cold objects are
- Temperature also determines the direction in which thermal energy will flow between two objects (or between an object and its surroundings)
- When thermal energy is exchanged, the objects (or systems) involved are said to have a thermal interaction
- The thermal energy exchanged during a thermal interaction is referred to as heat
- During a thermal interaction:
- Thermal energy always flows from the hotter object to the colder object
- The energy transfer continues until the two objects are in thermal equilibrium (i.e. they both have the same temperature)
- Thermal energy can be transferred via conduction, convection or radiation
- Temperature is a scalar quantity and it is measured using a thermometer
- It is measured in degrees Celsius (°C) or kelvin (K)
- The kelvin is the SI base unit for temperature
- The temperature of an object is a macroscopic measure of the average kinetic energy of the particles (atoms or molecules) that make up the object
Absolute temperature
- Absolute temperature is temperature measured in kelvin (K)
- Absolute zero is a temperature of zero kelvin (0 K) and corresponds to the temperature at which the average kinetic energy of the molecules is at its minimum
- The conversion between the Kelvin and the Celsius scale is given by:
T(K) = T(°C) + 273.15
- It is important to notice that differences in absolute temperatures correspond to differences in Celsius temperatures
ΔT(K) = ΔT(°C)
-
- Where ΔT stands for temperature change
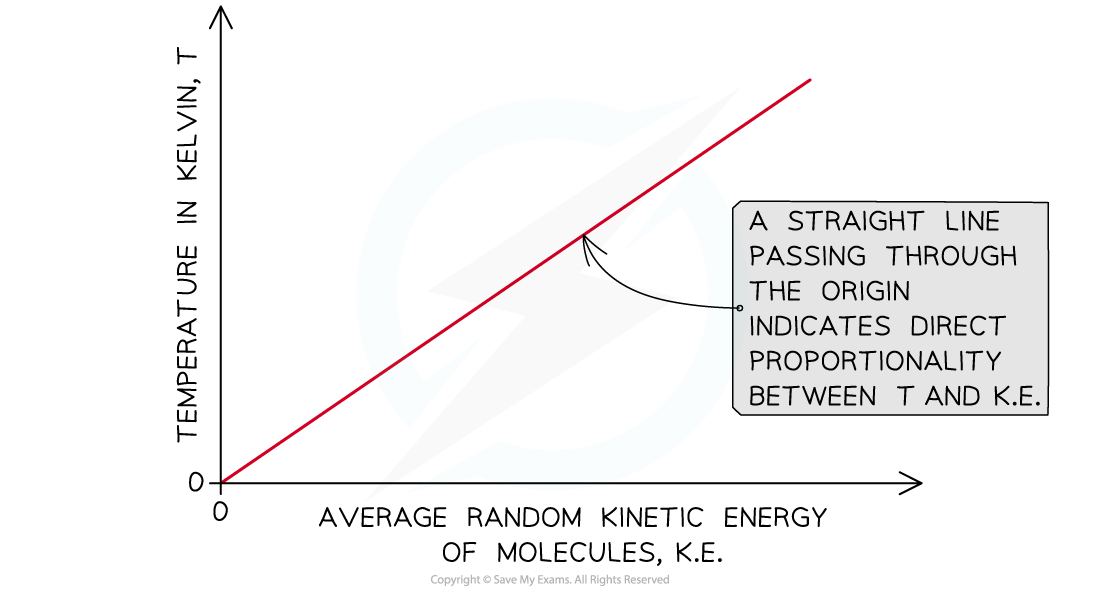
- The absolute temperature of a body is directly proportional to the average kinetic energy of the molecules within the body
The ice point is determined by placing a thermometer in a beaker containing melting ice, while the steam point is determined by placing the thermometer in a beaker with boiling water
Worked Example
Give an estimate of room temperature in kelvin (K).
Step 1: State a reasonable value for room temperature in degree Celsius (°C)
room temperature (°C) ~ 20°C
Step 2: Write down the conversion between Celsius scale and Kelvin scale
T(K) = T(°C) + 273.15
Step 3: Convert the room temperature value and express it in kelvin (K)
room temperature (K) ~ 293 K
Exam Tip
Remember that the lowest possible temperature on the Kelvin scale is absolute zero (0 K). Therefore, if you are calculating temperature in kelvin and you end up with a negative number, you need to check your work, since negative numbers do not exist on the Kelvin scale.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





