- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记11.1.1 Index of Hydrogen Deficiency
Index of Hydrogen Deficiency
- The degree of unsaturation or index of hydrogen deficiency provides information about the number of double or triple bonds in a molecule
- The IHD is the number of hydrogen molecules, H2, needed to convert the molecule to the corresponding saturated, non-cyclic molecule
- There are two ways to solve IHD problems. One way is to draw the structure and identify rings and double and triple bonds, counting each one as an IHD value of 1.
- The second way is to use a formula,
- For a compound containing CxHy, IHD= (2x+2-y)/2
- This is a little complicated, since for the formula to work you need to:
- ignore O and S
- count halogens as hydrogen
- add one C and one H for every nitrogen in the formula
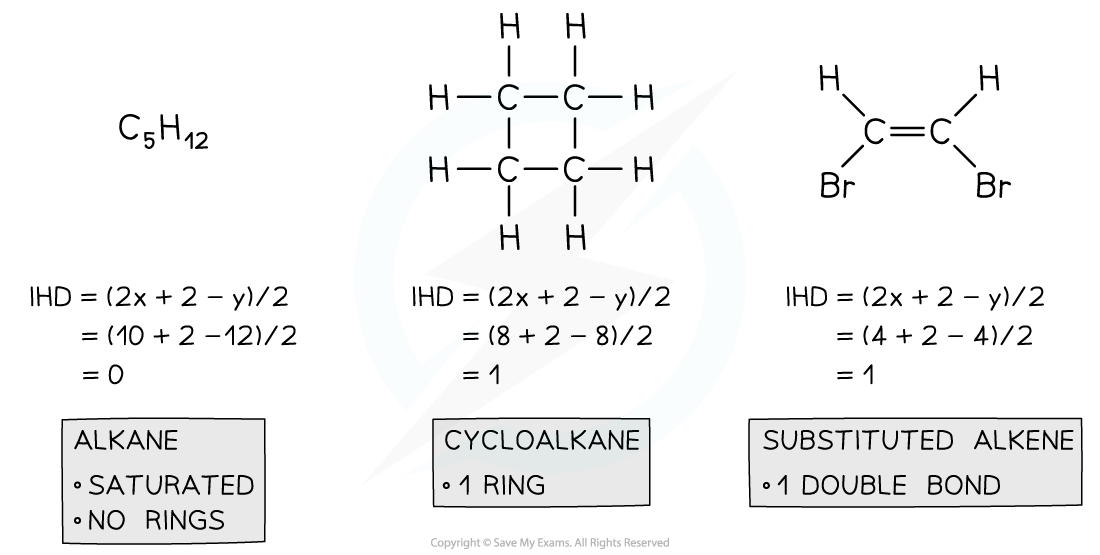
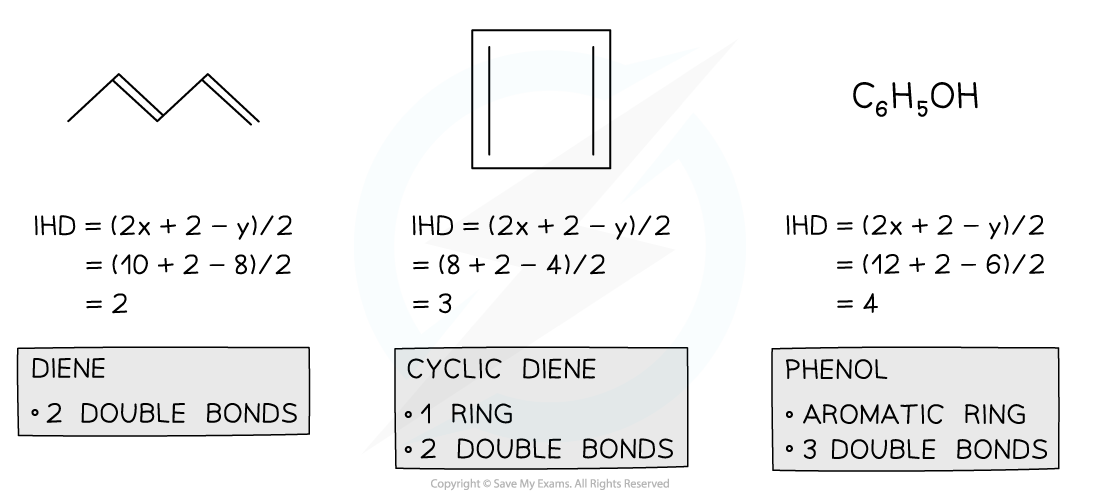
Index of Hydrogen Deficiency Examples
Worked Example
Deduce the Index of Hydrogen Deficiency in ethyne, C2H2, methyl benzene, C6H5CH3, and propanone, CH3COCH3
Answer:
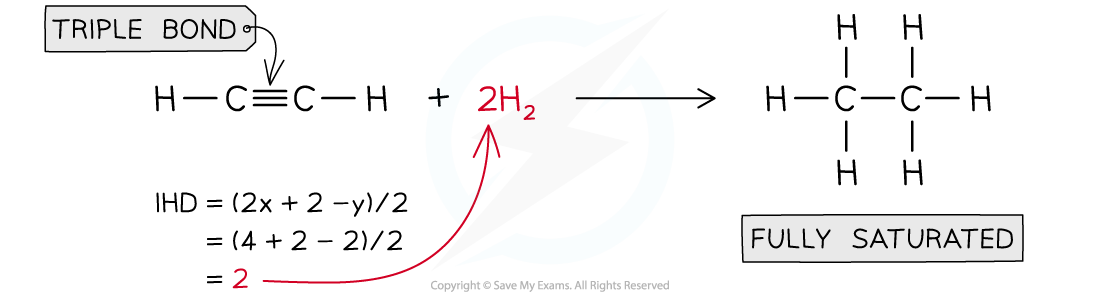
Answer 1: Ethyne, C2H2 IHD= 2
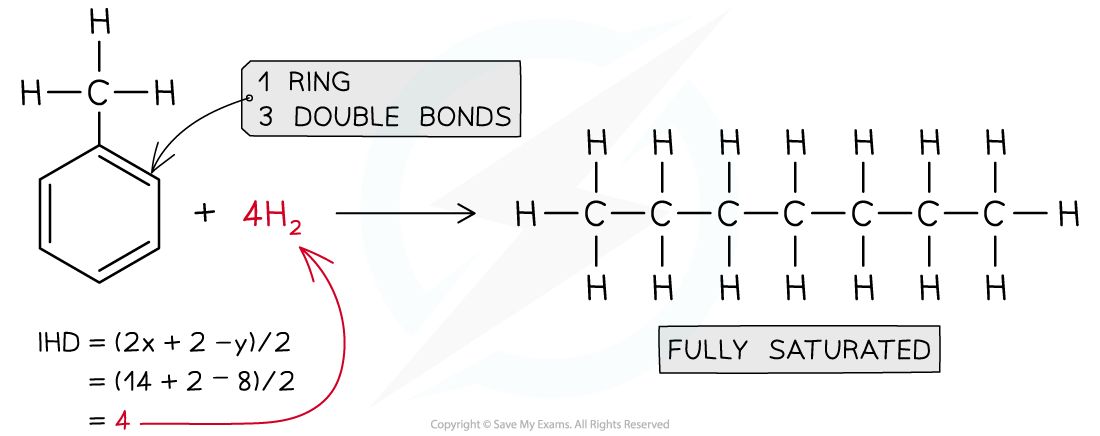
Answer 2: Methylbenzene, C6H5CH3, IHD= 4
Answer 3: Propanone,CH3COCH3 IHD = 1

Exam Tip
Drawing out the structure is a much faster way to solve IHD problems, but using the formula is helpful when you are struggling to draw the structure
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





