- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记10.2.12 Halogenoalkanes
Reactions of Halogenoalkanes
- Halogenoalkanes are much more reactive than alkanes due to the presence of the electronegative halogens
- The halogen-carbon bond is polar causing the carbon to carry a partial positive and the halogen a partial negative charge
- A nucleophilic substitution reaction is one in which a nucleophile attacks a carbon atom which carries a partial positive charge
- An atom that has a partial negative charge is replaced by the nucleophile
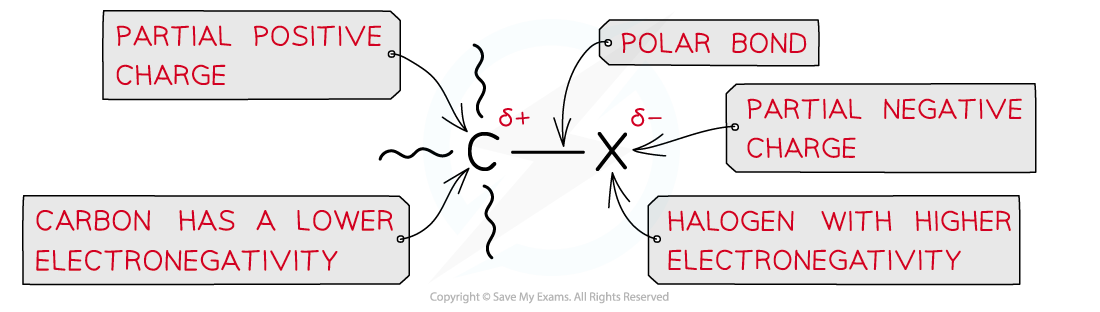
Due to large differences in electronegativity between the carbon and halogen atom, the C-X bond is polar
Reaction with NaOH
- The reaction of a halogenoalkane with aqueous alkali results in the formation of an alcohol
- The halogen is replaced by the OH-
- The aqueous hydroxide (OH- ion) behaves as a nucleophile by donating a pair of electrons to the carbon atom bonded to the halogen
- Hence, this reaction is a nucleophilic substitution
- For example, bromoethane reacts with aqueous alkali when heated to form ethanol

The halogen is replaced by a nucleophile, OH-
- The reaction is slow at room temperature so to ensure a high yield it is heated under reflux
- Since haloalkanes are not usually soluble in water, a polar solvent such as ethanol is often used as it will dissolve haloalkanes as well as sodium hydroxide
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





