- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记10.2.10 Alcohols - Oxidation
Oxidation of Primary Alcohols
- The products of oxidation of alcohols depends on the class of alcohols
- Here is a reminder of the three classes of alcohols:
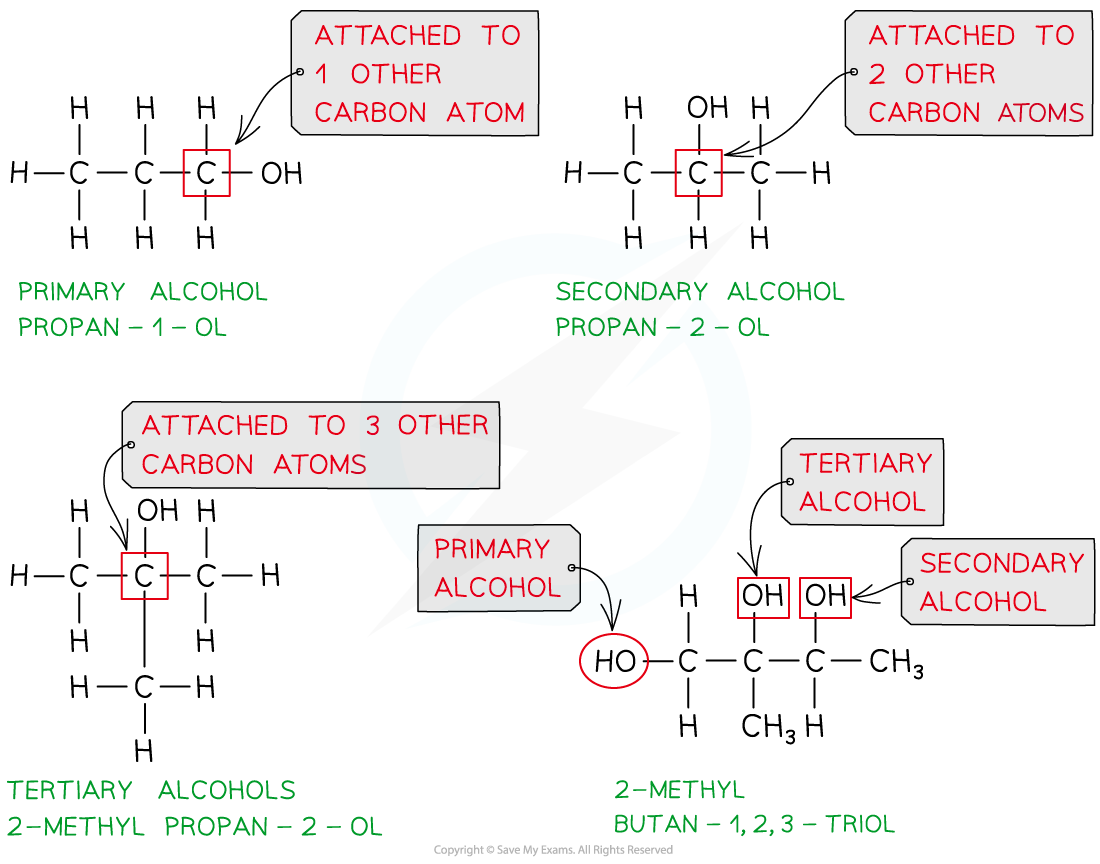 The three classes of alcohols
The three classes of alcohols
Primary alcohols
- Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
- The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
- Acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
- Acidified means that that the potassium dichromate(VI) is in a solution of dilute acid (such as dilute sulfuric acid)
- For potassium dichromate(VI) to act as an oxidising agent, it itself needs to be reduced
- When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
- Acidified potassium manganate(VII), KMnO4, is a purple oxidising agent
- As with acidified KMnO4 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
- When alcohols are oxidised, the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
- As with acidified KMnO4 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
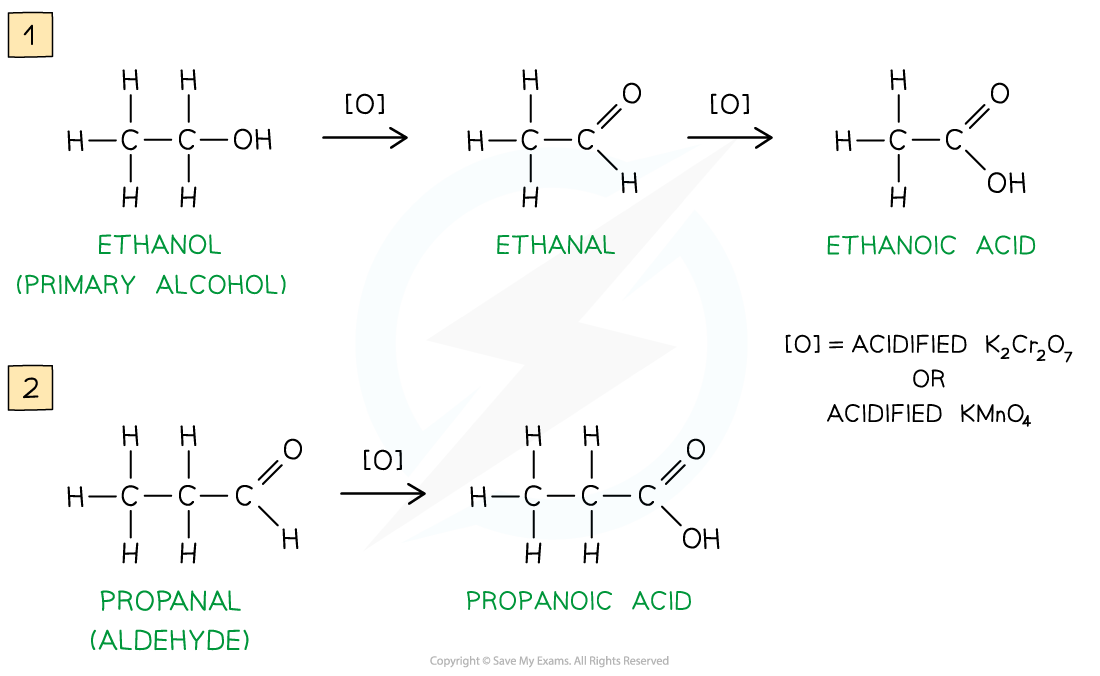
Further Oxidation
- If the aldehyde is not distilled off, further oxidation with excess oxidising agent will oxidise it to a carboxylic acid
- The reaction takes some time to complete and requires sustained heating
Test for alcohols
- The oxidation using acidified dichromate provides the basis for the test for alcohols as the reaction gives a strong colour change from orange to green
- Unfortunately, it does not work for tertiary alcohols, which cannot be oxidised
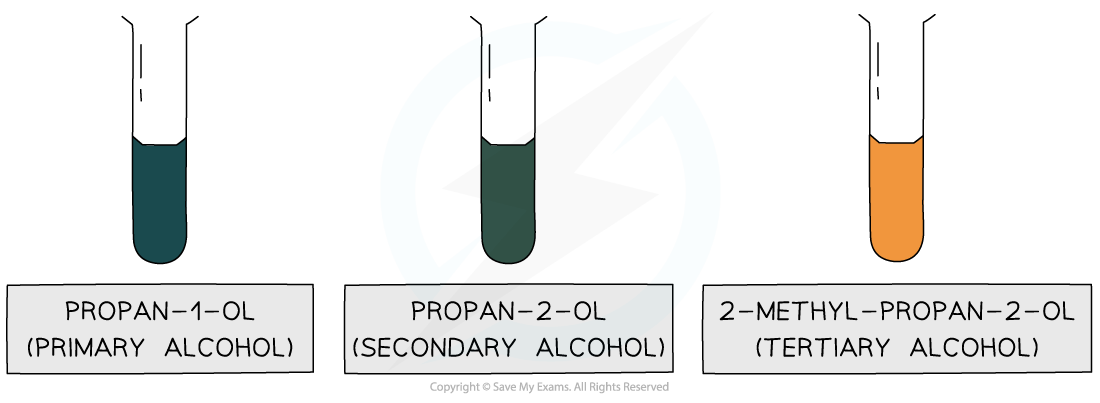
The test for primary and secondary alcohols
Oxidation of Secondary Alcohols
- Secondary alcohols can be oxidised to form ketones only
- To get a good yield of the ketone the reaction requires some sustained heating
 Oxidation of Secondary Alcohols
Oxidation of Secondary Alcohols
- Tertiary alcohols do not undergo oxidation
- This is because there must be a hydrogen on the functional group carbon, which breaks off to form water
- There are only C-C bonds on the functional group carbon in a tertiary alcohol
Distillation & Reflux
The difference between using distillation and heating under reflux
- To produce an aldehyde from a primary alcohol the reaction mixture must be heated
- The aldehyde product has a lower boiling point than the alcohol ( since it has lost the H-bonding) so it can be distilled off as soon as it forms
- Distillation can be carried out using a simple side arm arrangement which acts as an air condenser or the vapours can be made to pass through a condenser
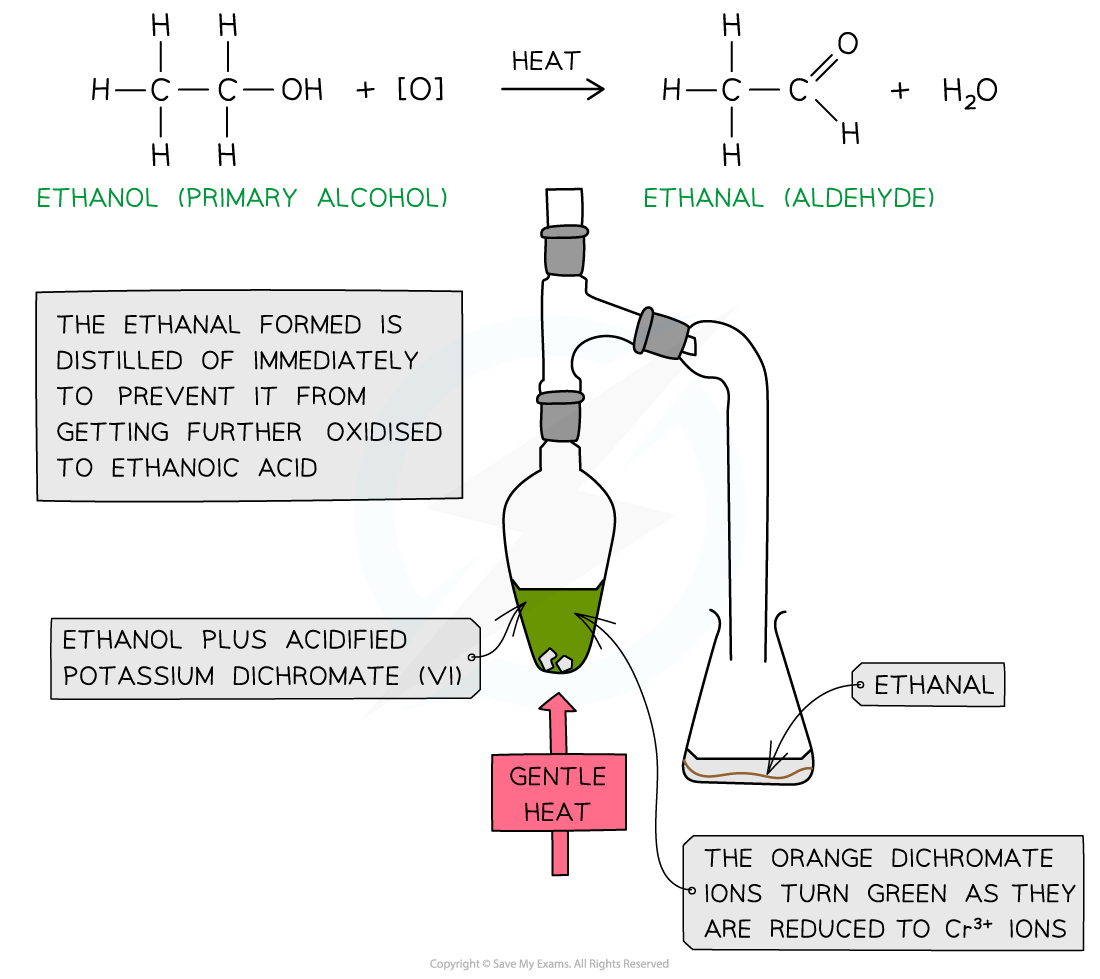
Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation
Heating under reflux
- For reactions that require sustained heating the apparatus has to be modified
- To prevent loss of volatile reactants the apparatus includes a condenser in the vertical position which returns components back into the reaction flask
- This is known as heating under reflux (reflux means re-boiling)
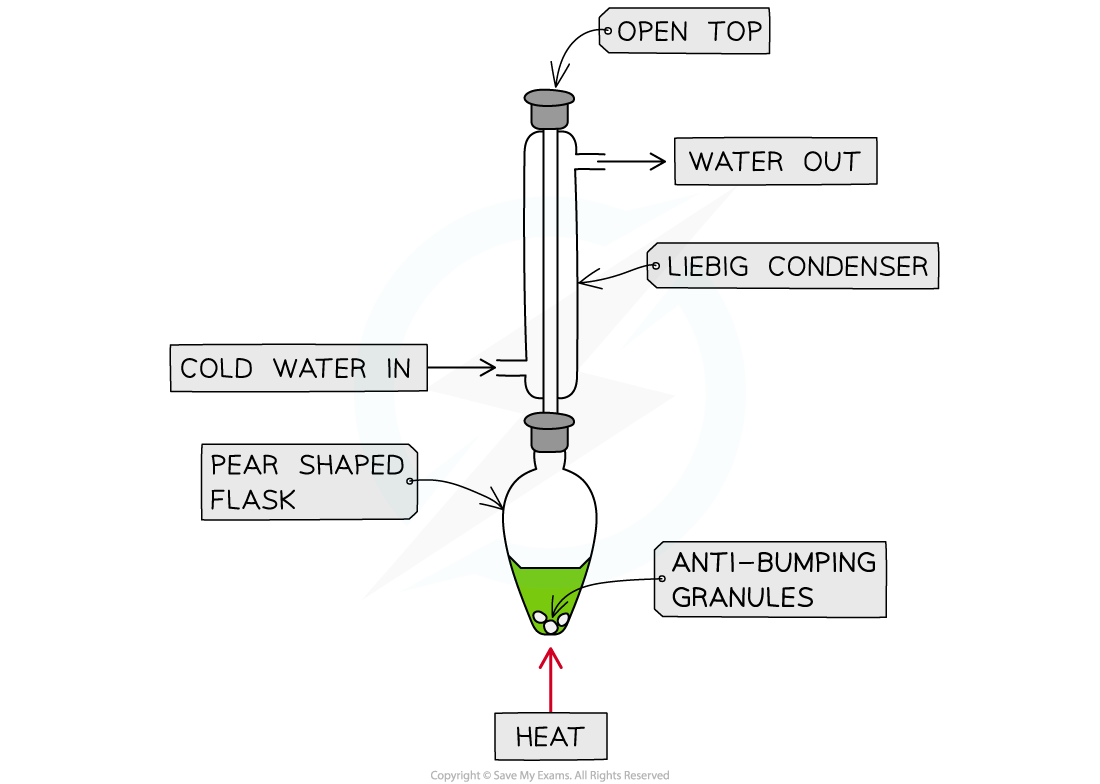
Heating under Reflux Apparatus
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





