- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记10.1.9 Organic Families - Organic Nitrogen Compounds
Organic Nitrogen Compounds
Amines
- There are three organic nitrogen families that you need to know: amines, amides and nitriles
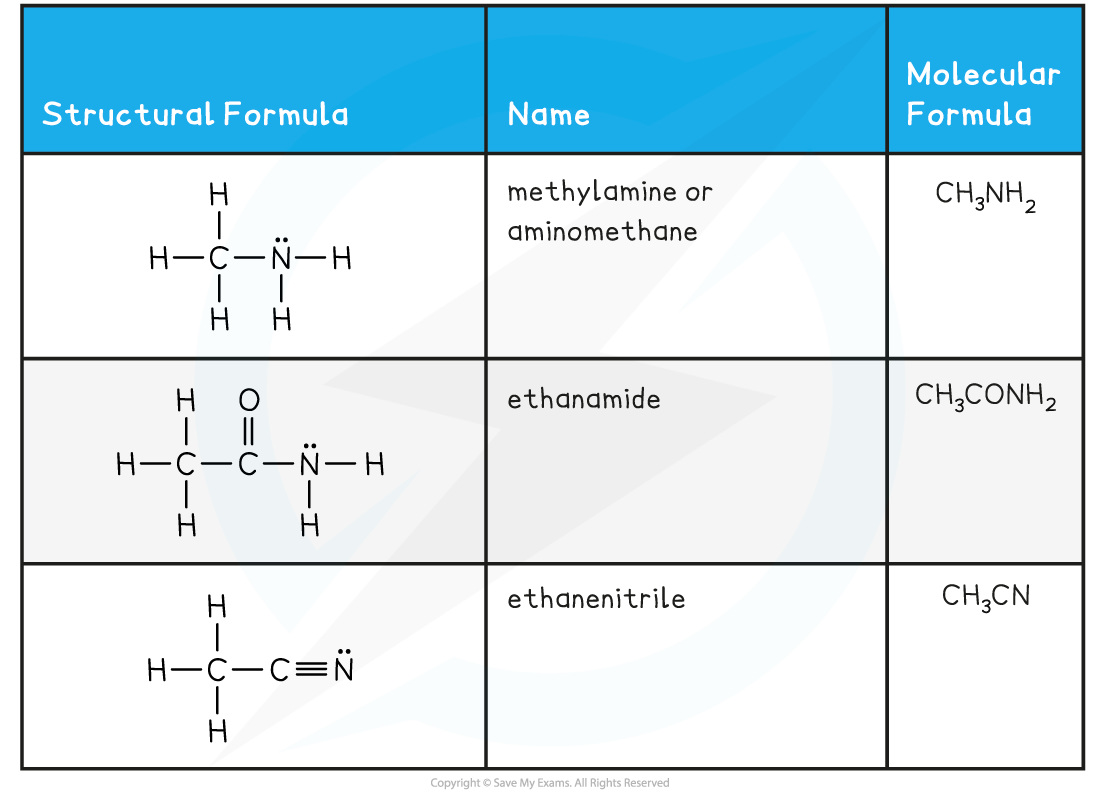
- Amine is the name given to compounds containing the functional group amino, -NH2
- Amines are derived from ammonia where one H in ammonia (NH3)has been replaced by an R (alkyl) group
- The general formula of an amine is CnH2n+1NH2 which can be shortened to just RNH2
- The nomenclature of an amine follows the pattern alkyl + amine or amino + alkane
- E.g. propylamine or aminopropane
Amides
- Amide is the name given to compounds containing the functional group carboxamide, -CONH2
- Amides are a combination of amino and carbonyl groups
- The general formula of an amide is CnH2n+1CONH2 which can be shortened to just RCONH2
- The nomenclature of an amide follows the pattern alkan + amide
Nitriles
- Nitriles are compounds containing the functional group nitrile, -CN
- This is the same CN group that is called a cyanide group as an ion, just as hydroxyl group, OH is called hydroxide in inorganic chemistry
- The general formula of an nitrile is CnH2n+1CN which can be shortened to just RCN
- The nomenclature of a nitrile follows the pattern alkane + nitrile (some people leave out the 'e' on the alkane)
- E.g ethanenitrile or ethannitrile
Organic Nitrogen Compounds Examples
Exam Tip
Be careful about counting all the carbons when naming a nitrile. For example C3H7CN is butanenitrile not propanenitrile as the longest chain is 4 carbons.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





