- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记10.1.8 Organic Families - Carboxylic Acids & Esters
Carboxylic Acids & Esters
Carboxylic acids
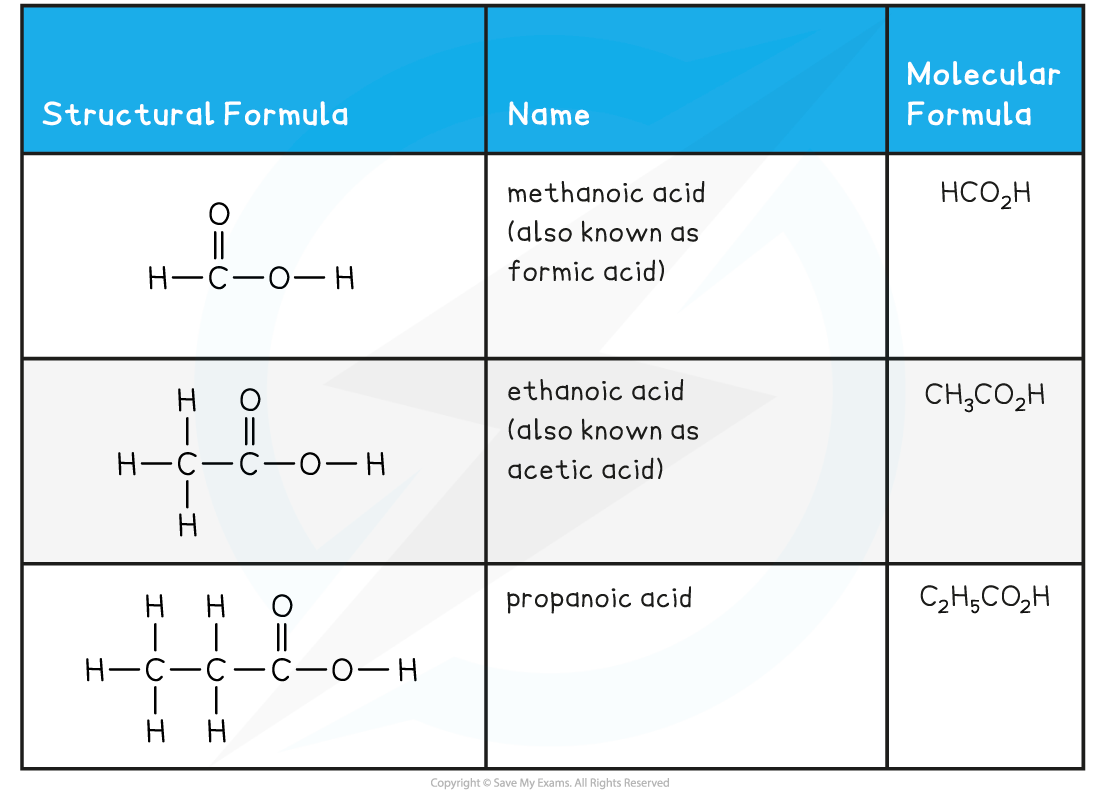
- Carboxylic acids is the name given to compounds containing the functional group carboxyl, -COOH
- The general formula of a carboxylic acid is CnH2n+1COOH which can be shortened to just RCOOH
- (In some countries the family is called alkanoic acid)
- The nomenclature of carboxylic acid follows the pattern alkan + oic acid
- There is no need to use numbers in the name as the carboxyl group will always be on the number 1 carbon atom
Carboxylic Acids Examples Table
Esters
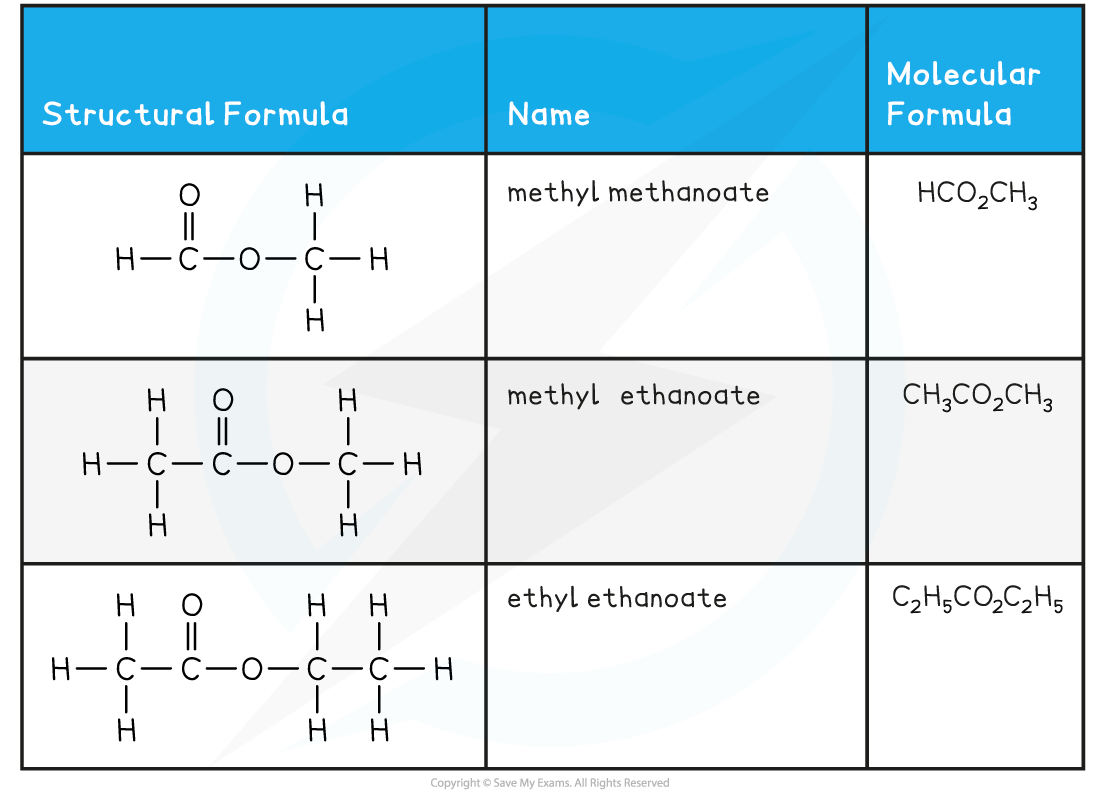
- Esters are functional group isomers of carboxylic acids and contain the functional group, carboxylate, -COOR
- The general formula of an ester is usually represented as RCOOR where R can be the same or different on either side of the carboxylate group
- The nomenclature of esters follows the pattern alkyl + alkanoate
- The alkyl group in the name is the R group attached to the oxygen
Esters Examples Table
- Carboxylic acids and esters contain few similarities in their chemical and physical properties
- H-bonds are present between carboxylic acid molecules and not between esters, so this affects the melting point, boiling point and solubility:
- Smaller chain carboxylic acids are soluble in water and have higher boiling points than expected (e.g. ethanoic acid is 117 oC)
- Esters are insoluble in water and have lower boiling points than their isomeric carboxylic acids ( e.g. methyl methanoate is 31 oC)
Exam Tip
The C in RCOOR is included in the name of the first R group, so C3H7COOCH3 is methyl butanoate not methyl propanoate.Don't be fooled by the order of the atoms in the linear formula: CH3OC(O)C3H7 is also an acceptable way to write the formula of methyl butanoate!
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





