- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记10.1.1 Homologous Series
Homologous Series
- Organic chemistry is the chemistry of carbon compounds
- Carbon forms a vast number of compounds because it can form strong covalent bonds with itself
- This enables it to form long chains of carbon atoms, and hence an almost infinite variety of carbon compounds are known
- The tendency of identical atoms to form covalent bonds with each other and hence form chains is known as catenation

Catenation in carbon allows an almost infinite variety of chains, branches and rings
- Carbon always forms four covalent bonds which can be single, double or triple bonds
- A functional group is a specific atom or group of atoms which confer certain physical and chemical properties onto the molecule
- Organic molecules are classified by the dominant functional group on the molecule
- Organic compounds with the same functional group, but a different number of carbon atoms, are said to belong to the same homologous series
- Every time a carbon atom is added to the chain, two hydrogen atoms are also added
Homologous Series of Alkanes Table
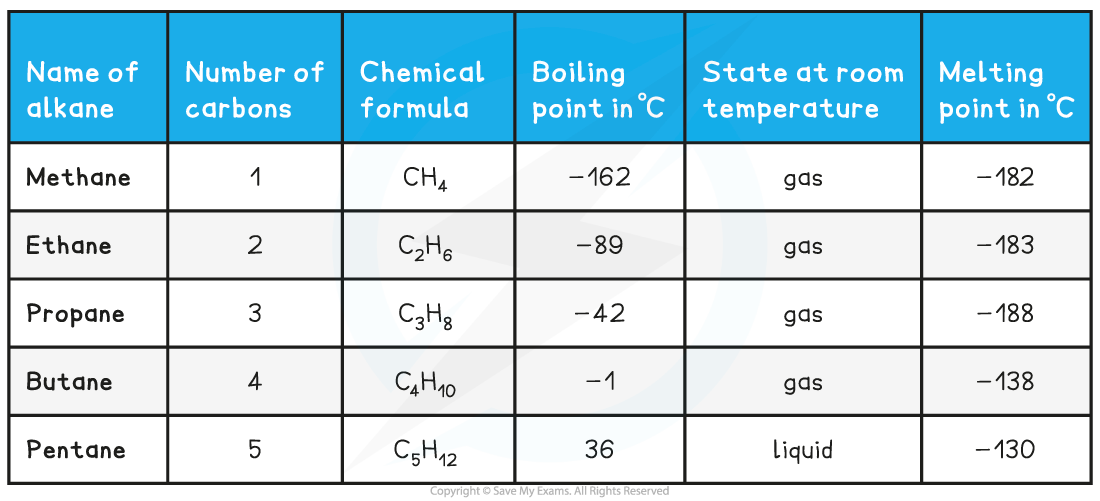
- Things we can say about a homologous series:
- each member has the same functional group
- each member has the same general formula
- each member has similar chemical properties
- each member differs by -CH2 -
- members have gradually changing physical properties, for example, boiling point, melting point and density
- As a homologous series is ascended, the size of the molecule increases
- This has an effect on the physical properties, such as boiling point and density
Boiling Point Trends
- A graph of boiling point for the first eight alkanes looks like this:
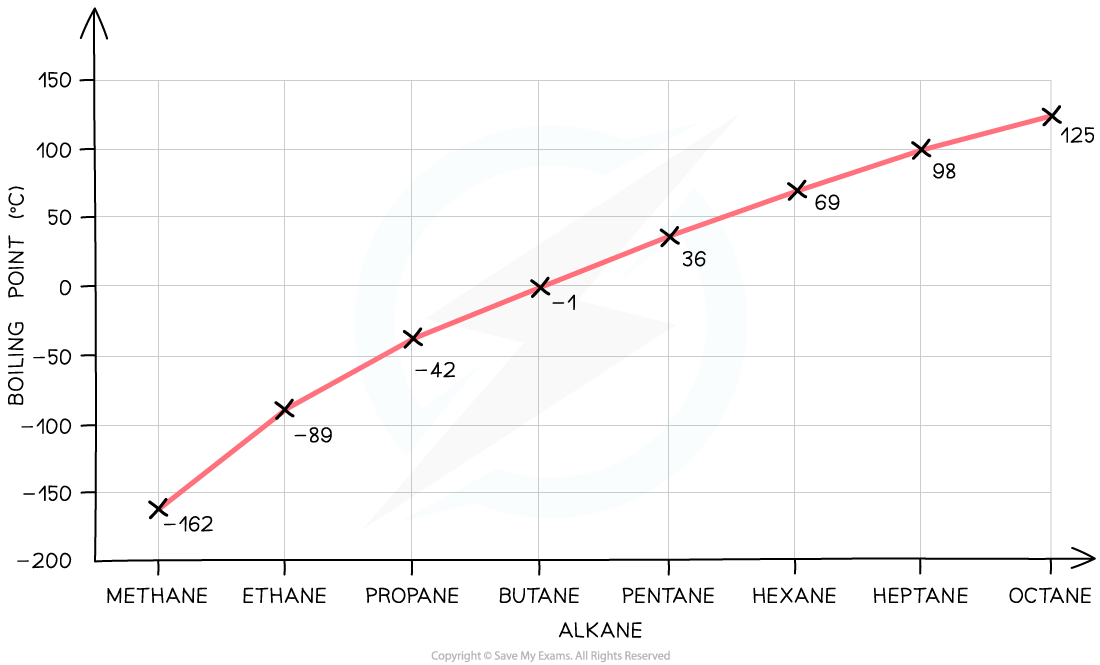
- The broad trend is that boiling point increases with increased molecular size
- Each additional -CH2 - (called the homologous increment ) adds 8 more electrons to the molecule
- This increases the strength of the London Dispersion Forces
- Stronger LDF leads to a higher boiling point
- These trends are followed in other homologous series
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





