- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记8.2.5 Acid-Base Calculations
Acid-Base Calculations
- Using the relationships between pH, [H⁺] and [OH⁻] a variety of problems can be solved
pH = - log [H+] and Kw = [H+] [OH-]
- Test your understanding on the following worked examples:
Worked Example
- The pH of a solution of phosphoric acid changes from 3 to 5. Deduce how the hydrogen ion concentration changes
- Water from a pond was analysed and found to have a hydrogen ion concentration of 2.6 x 10-5 mol dm-3. Calculate the pH of the pond water.
- Determine the pH of a solution made by dissolving 5.00 g of potassium hydroxide in 250 cm3 of distilled water
Answers:
Answer 1: The initial pH of the phosphoric acid is 3 which corresponds to a hydrogen ion concentration of 1 x 10-3 mol dm-3 :
[H+] = 10-pH
[H+] = 1 x 10-3 mol dm-3
The final pH is 5, which corresponds to 1 x 10-5 mol dm-3
Therefore, the solution has decreased in [H+] concentration by 102 or 100 times
Answer 2: The pond water has [H+] = 2.6 x 10-5 mol dm-3.
pH = - log [H+] = -log(2.6 x 10-5) = 4.58
Answer 3: Potassium hydroxide (M = 56.10 g mol-1) is a strong base so the concentration of [OH-] is the same as the concentration of the solution as it fully dissociates:
KOH (s) → K+ (aq) + OH- (aq)
The concentration of KOH is

Using Kw = [H+][OH-], and then rearranging [H+] = Kw /[OH-]
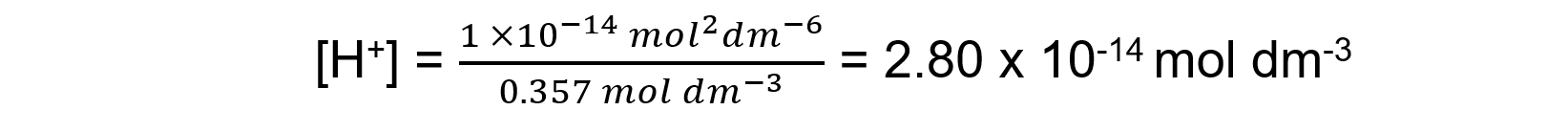
pH = - log (2.80 x 10-14) = 13.55
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





