- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记8.1.4 Neutralization
Neutralization
- A neutralisation reaction is one in which an acid (pH <7) and a base/alkali (pH >7) react together to form water (pH = 7) and a salt

- The proton of the acid reacts with the hydroxide of the base to form water

- The spectator ions which are not involved in the formation of water, form the salt
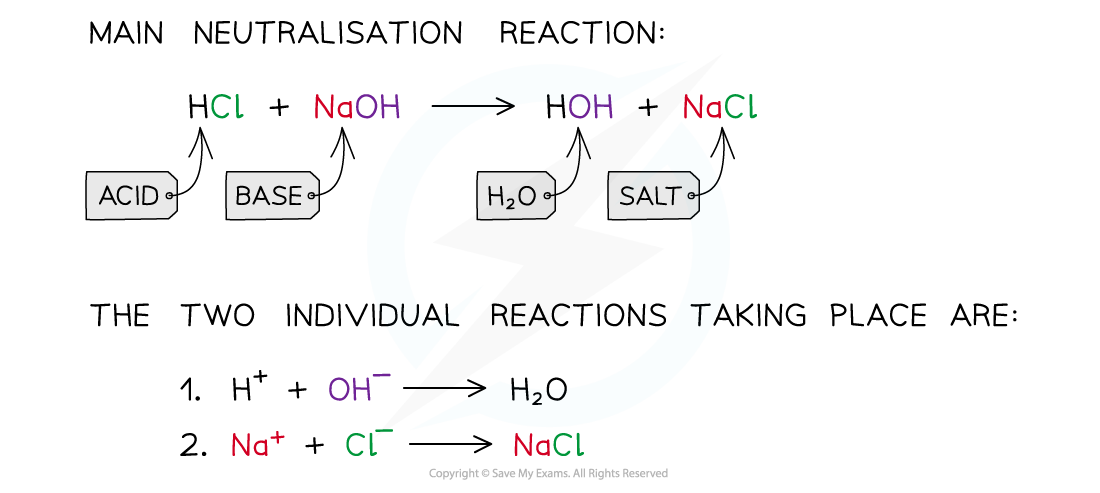
The diagram shows a neutralisation reaction of HCl and NaOH and the two individual reactions that take place to form the water and salt
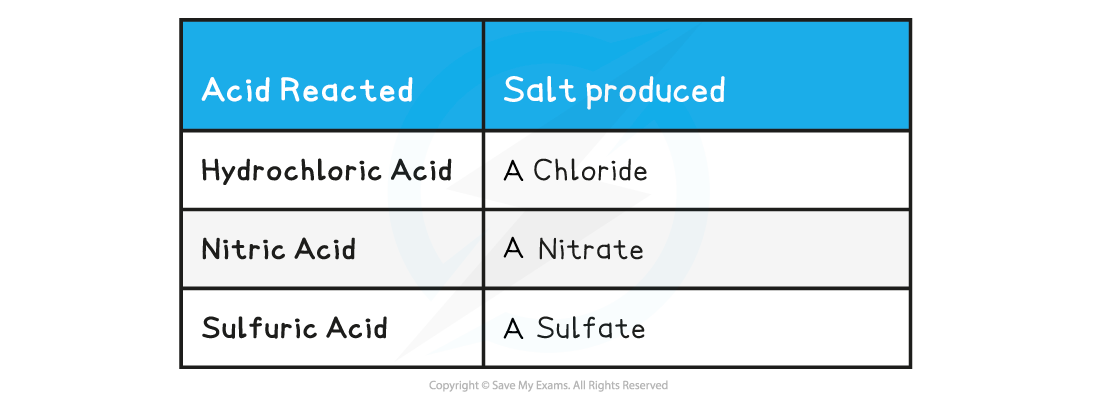
- The name of the salt produced can be predicted from the acid that has reacted
Acid Reacted & Salt Table
Exam Tip
The enthalpy of neutralisation is the enthalpy change that occurs when an acid reacts with a base to form one mole of water. Since the reaction between strong acids and strong bases is the same regardless of the acid or base, it should be no surprise the enthalpy change is the same and is approximately -57 kJ mol-1
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





