- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.3.2 Deducing Intermolecular Forces
Deducing Intermolecular Forces
- In order to deduce the types of intermolecular forces present in molecules you need information about the structure and chemical formula of the molecules
- The chemical formula will tell you about the presence of electronegative elements present in the molecule
- Any potential polar bonds can be identified
- If N, O or F is present then hydrogen bonds are potentially possible
- The structure and symmetry of the molecule will enable you to determine if the molecule is polar following the principles laid out in 4.1.10 Molecular Polarity
Worked Example
Which of the compounds below can form intermolecular hydrogen bonds in the liquid state?
A. (CH3CH2)3N
B. CH3OCH3
C. CCl4
D. C2H5OH
Answer:
The correct option is D.
-
- Draw the displayed structures of the molecules:
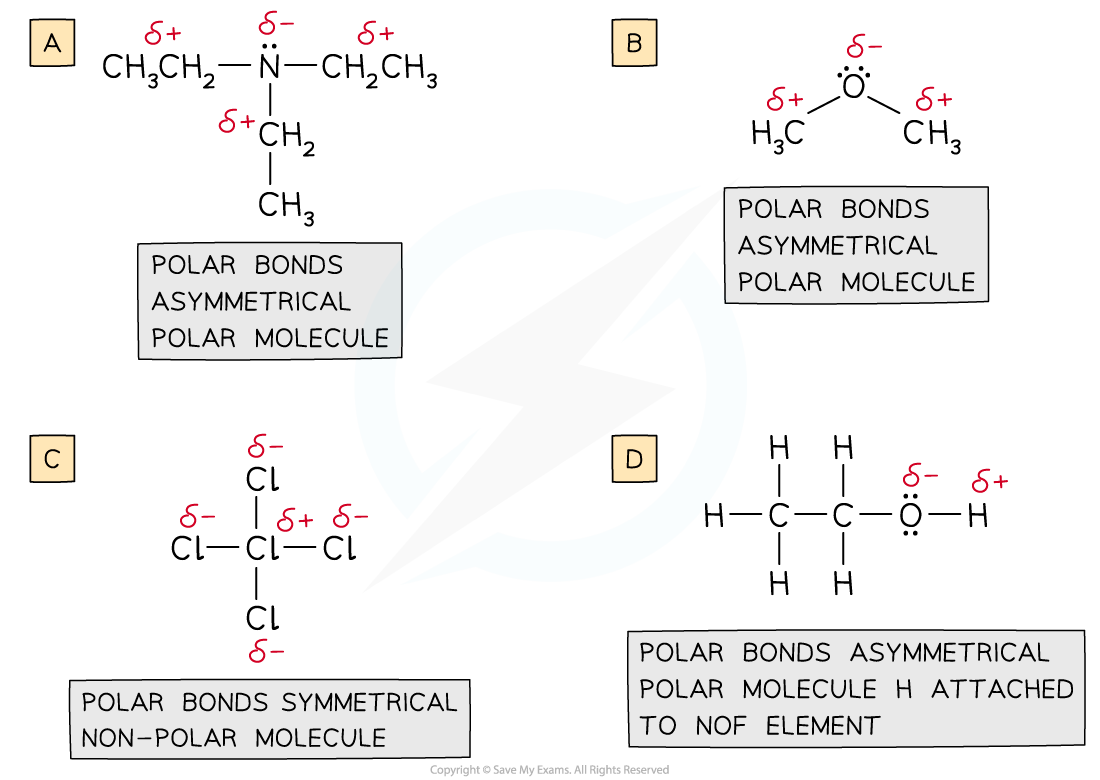
-
- Molecules A and B form dipole-dipole attractions as they are polar molecules
- Molecule C forms dispersion forces as the dipoles cancel out so there is no overall polarity
- Molecule D is the only one capable of forming hydrogen bonds
Exam Tip
Sometimes a question will ask you to name all the IMFs present in molecules and students frequently forget to include dispersion forces which are present in all molecules, since everything contain electrons!
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





