- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.1.6 Lewis Structures
Lewis Structures
- Lewis structures are simplified electron shell diagrams and show pairs of electrons around atoms.
- A pair of electrons can be represented by dots, crosses, a combination of dots and crosses or by a line. For example, chlorine can be shown as:
 Different Lewis Structures for chlorine molecules
Different Lewis Structures for chlorine molecules
- Note: Cl–Cl is not a Lewis structure, since it does not show all the electron pairs.
- The “octet rule” refers to the tendency of atoms to gain a valence shell with a total of 8 electrons
Steps for drawing Lewis Structures
-
- Count the total number of valence
- Draw the skeletal structure to show how many atoms are linked to each other.
- Use a pair of crosses or dot/cross to put an electron pair in each bond between the atoms.
- Add more electron pairs to complete the octets around the atoms ( except H which has 2 electrons)
- If there are not enough electrons to complete the octets, form double/triple bonds.
- Check the total number of electrons in the finished structure is equal to the total number of valence electrons
Worked Example
Draw a Lewis structure for CCl4
Answer:
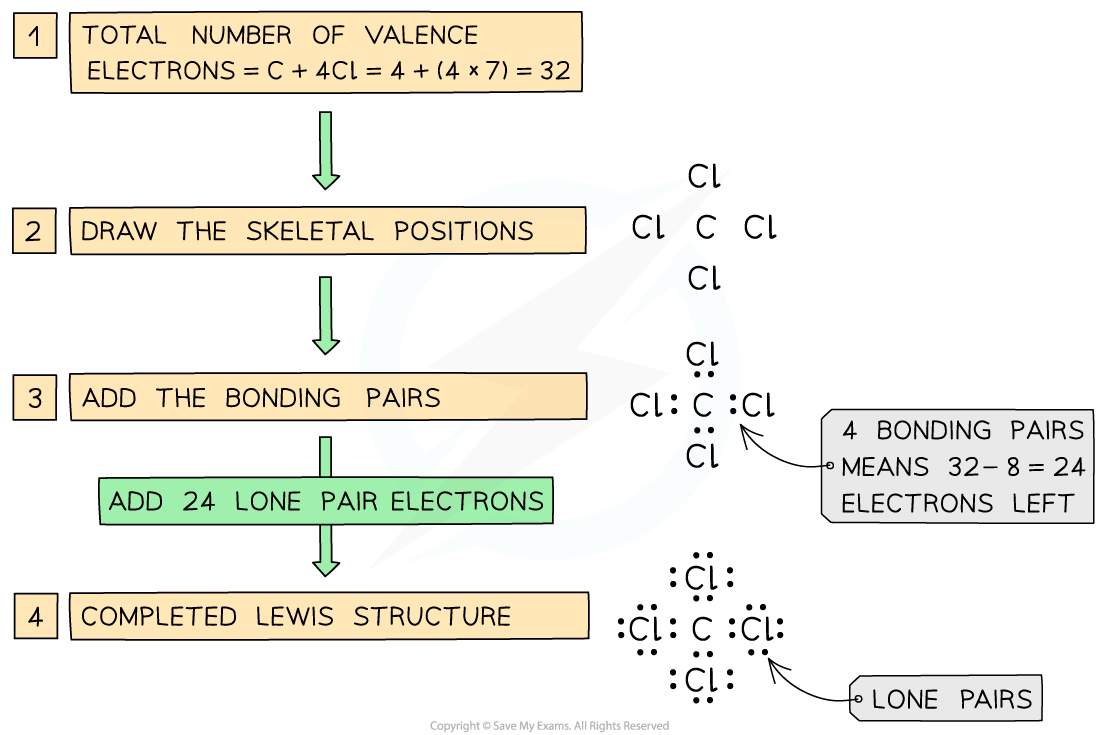
Steps in drawing the Lewis Structure for CCl4
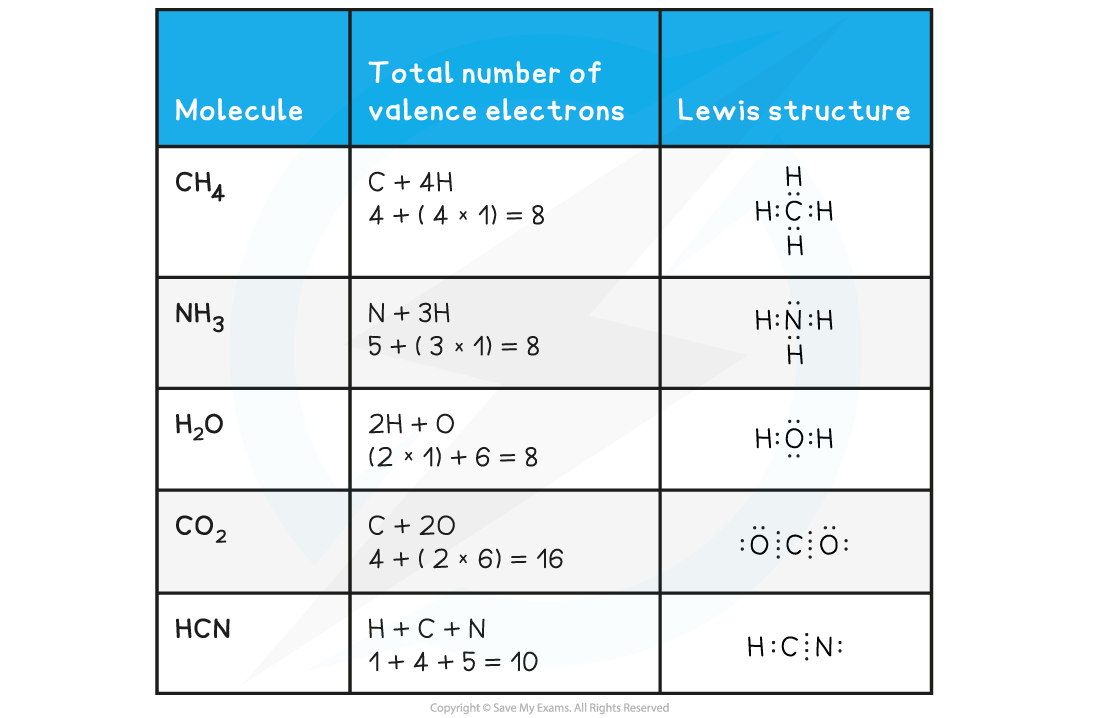
Further examples of Lewis structures
- Follow the steps for drawing Lewis structures for these common molecules
Incomplete Octets
- For elements below atomic number 20 the octet rule states that the atoms try to achieve 8 electrons in their valence shells, so they have the same electron configuration as a noble gas
- However, there are some elements that are exceptions to the octet rule, such a H, Li, Be, B and Al
- H can achieve a stable arrangement by gaining an electron to become 1s2, the same structure as the noble gas helium
- Li does the same, but losing an electron and going from 1s22s1 to 1s2 to become a Li+ ion
- Be from group 2, has two valence electrons and forms stable compounds with just four electrons in the valence shell
- B and Al in group 13 have 3 valence electrons and can form stable compounds with only 6 valence electrons
- There are two examples of Lewis structures with incomplete octets you should know, BeCl2 and BF3:
Incomplete Octets Examples

- Test your understanding of Lewis diagrams in the following example:
Worked Example
How many electrons are in the 2-aminoethanoic acid molecule?
A. 18
B. 20
C. 28
D. 30
Answer:
The correct option is D.
-
- You must count the lone pairs on N and O as well as the bonding pairs. There are 5 ‘hidden’ pairs of bonding electrons in the OH, CH2 and NH2 groups. Hydrogen does not follow the octet rule.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1