- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: HL复习笔记12.2.1 Rutherford Scattering & Nuclear Radius
Rutherford Scattering & Nuclear Radius
- In the Rutherford scattering experiment, alpha particles are fired at a thin gold foil
- Initially, before interacting with the foil, the particles have kinetic energy,
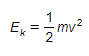
- Some of the alpha particles are found to come straight back from the gold foil
- This indicates that there is electrostatic repulsion between the alpha particles and the gold nucleus
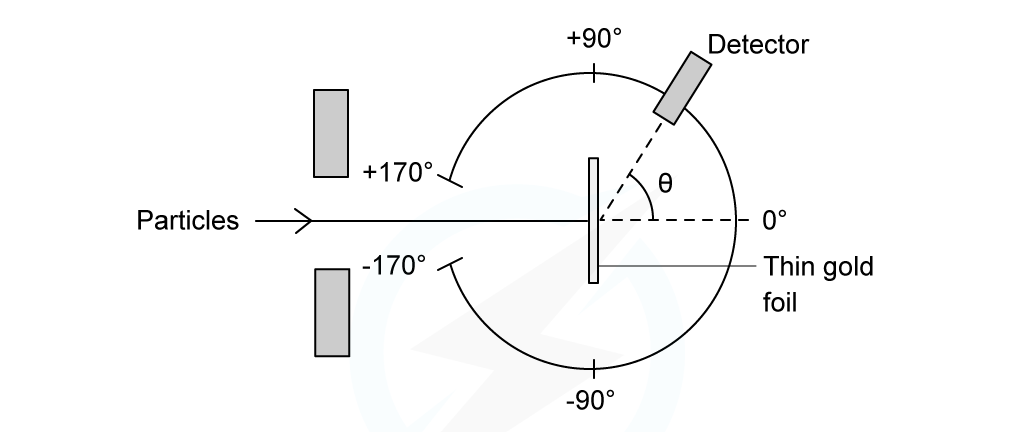
Experimental set up of the Rutherford alpha scattering experiment
- At the point of closest approach, d, the repulsive force reduces the speed of the alpha particles to zero momentarily, before any change in direction
- At this point, the initial kinetic energy of an alpha particle, Ek, is equal to electric potential energy, Ep
- The radius of the closest approach can be found be equating the initial kinetic energy to the electric potential energy
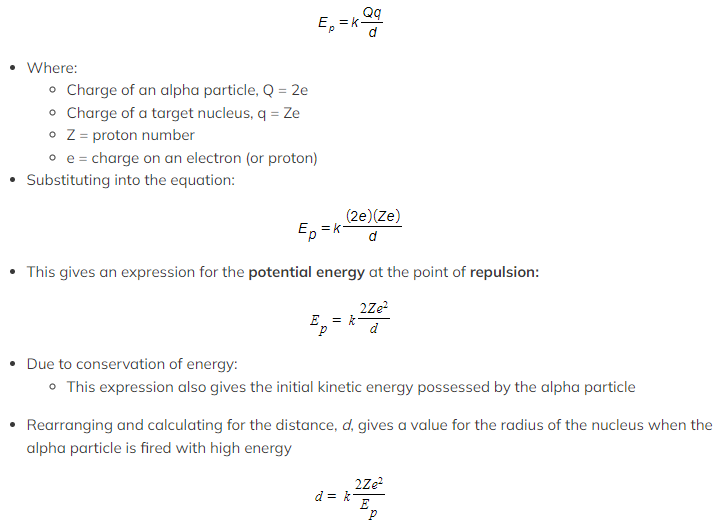

The closest approach method of determining the size of a gold nucleus
Nuclear Radius
- The radius of nuclei depends on the nucleon number, A of the atom
- This makes sense because as more nucleons are added to a nucleus, more space is occupied by the nucleus, hence giving it a larger radius
- The exact relationship between the radius and nucleon number can be determined from experimental data
- By doing this, physicists were able to deduce the following relationship:

- Where:
- R = nuclear radius (m)
- A = nucleon / mass number
- R0 = constant of proportionality = 1.20 fm
Nuclear Density
- Assuming that the nucleus is spherical, its volume is equal to:
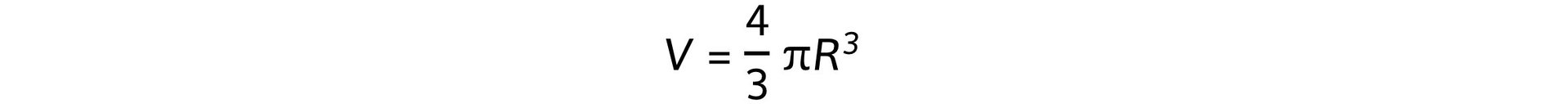
- Where R is the nuclear radius, which is related to mass number, A, by the equation:

- Where R0 is a constant of proportionality
- Combining these equations gives:
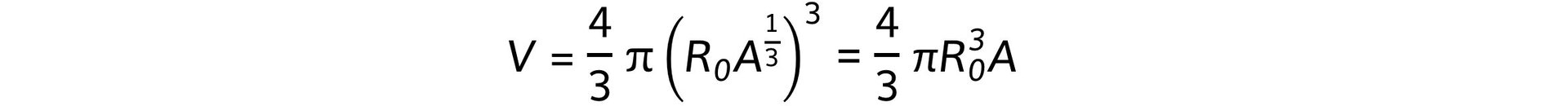
- Therefore, the nuclear volume, V, is proportional to the mass of the nucleus, A
- Mass (m), volume (V), and density (ρ) are related by the equation:
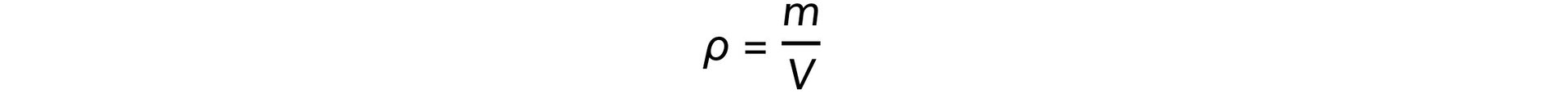
- The mass, m, of a nucleus is equal to:
m = Au
- Where:
- A = the mass number
- u = atomic mass unit
- Using the equations for mass and volume, nuclear density is equal to:
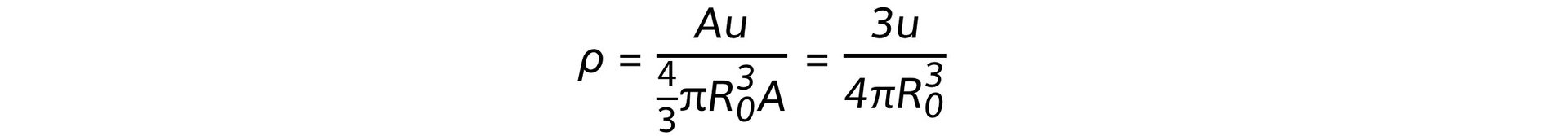
- Since the mass number A cancels out, the remaining quantities in the equation are all constant
- Therefore, this shows the density of the nucleus is:
- Constant
- Independent of the radius
- The fact that nuclear density is constant shows that nucleons are evenly separated throughout the nucleus regardless of their size
- The accuracy of nuclear density depends on the accuracy of the constant R0, as a guide nuclear density should always be of the order 1017 kg m–3
- Nuclear density is significantly larger than atomic density, this suggests:
- The majority of the atom’s mass is contained in the nucleus
- The nucleus is very small compared to the atom
- Atoms must be predominantly empty space
Worked Example
Determine the value of nuclear density.
You may take the constant of proportionality, R0, to be 1.20 × 10–15 m.
Step 1: Derive an expression for nuclear density
Using the equation derived above, the density of the nucleus is: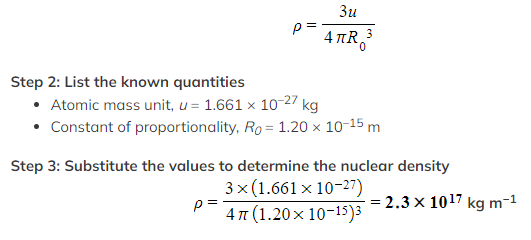
Exam Tip
Make sure you're comfortable with the calculations involved with the alpha particle closest approach method, as this is a common exam question.
You will be expected to remember that the charge of an α is the charge of 2 protons (2 × the charge of an electron)
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





