- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
2019USNCO考试大纲(翰林国际教育整理)
通用概念Concepts
- Estimation of experimental errors, use of significant figures;
- Nucleons, isotopes, radioactive decay and nuclear reactions (alpha, beta, gamma);
- Quantum numbers (n,l,m) and orbitals (s,p,d) in hydrogen-like atoms;
- Hund’s rule, Pauli principle;
- Electronic configuration of main group and the first row transition metal atoms and their ions;
- Periodic table and trends (electronegativity, electronaffinity, ionization energy, atomic and ionic size, melting points, metallic character, reactivity);
- Bond types (covalent, ionic, metallic), intermolecular forces and relation to properties;
- Molecular structures and simple VSEPR theory (up to 4 e pairs);
- Balancing equations, empirical formulae, mole concept and Avogadro’s number,stoichiometric calculations, density, calculations with different concentration units;
- Chemical equilibrium, Le Chatelier’s principle, equilibrium constants in terms of concentrations, pressures and mole fractions;
- Arrhenius and Bronsted acid-base theory, pH, self-ionization of water, equilibrium constants
of acid-base reactions, pH of weak acid solutions, pH of very dilute solutions and simple
buffer solutions, hydrolyis of salts; - Solubility constants and solubility;
- Complexation reactions, definition of coordination number, complex formation constants;
- Basics of electrochemistry: Electromotive force, Nernst equation; Electrolysis, Faraday’s laws;
- Rate of chemical reactions, elementary reactions, factors affecting the reaction rate, rate law for homogeneous and heterogeneous reactions, rate constant, reaction order, reaction energy profile, activation energy, catalysis, influence of a catalyst on thermodynamic and kinetic characteristics of a reaction;
- Energy, heat and work, enthalpy and energy, heat capacity, Hess’ law, standard formation
enthalpies, solution, solvation and bond enthalpies; - Definition and concept of entropy and Gibbs’ energy, second law of thermodynamics,
direction of spontaneous change; - Ideal gas law, partial pressures;
- Principles of direct and indirect tiration (back titration);
- Acidi and alkalimetry, acidimetric titration curves, choice and colour of indicators for
acidimetry; - Redox titrations (permanganometric and iodometric);
- Simple complexometric and precipitation titrations;
- Basic principles of inorganic qualitative analysis for ions specified in factual knowledge,
flame tests; - Lambert-Beer law;
- Organic structure-reactivity relations (polarity, electrophilicity, nucleophilicity, inductive effects, relative stability)
- Structure-property relations (boiling point, acidity, basicity);
- Simple organic nomenclature;
- Hybridization and geometry at carbon centers;
- Sigma and pi bonds, delocalization, aromaticity, mesomeric structures;
- Isomerism (constitutional, configuration, conformation, tautomerism)
- Stereochemistry (E-Z, cis-trans isomers, chirality, optical activity, Cahn-Ingold-Prelog
system, Fisher projections); - Hydrophilic and hydrophobic groups, micelle formation;
- Polymers and monomers, chain polymerizations, polyaddition and polycondensation;
实验技能Laboratory skills
- Heating in the laboratory, heating under reflux;
- Mass and volume measurement (with electronic balance, measuring cylinder, pipette and
burette, volumetric flask); - Preparation and dilution of solutions and standard solutions;
- Operation of a magnetic stirrer;
- Carrying out of test tube reactions;
- Qualitative testing for organic functional groups (using a given procedure);
- Volumetric determination, titrations, use of a pipette bulb;
- Measurement of pH (by pH paper or calibrated pH meter);
有机化学Organic:
- Common electrophiles and nucleophiles
- Electrophilic addition: addition to double and triple bonds, regioselectivity (Markovnikoff’s rule), stereochemistry
- Electrophilic substitution: substitution on aromatic rings, influence of substituents on the reactivity and regioselectivity, electrophilic species;
- Elimination: E1 and E2 reactions at sp3 carbon centers, stereochemistry, acid-base catalysis,
common leaving groups; - Nucleophilic substitution: SN1 and SN2 reactions at sp3 carbon centers, stereochemistry;
- Nucleophilic addition: addition to carbon-carbon and carbon-hetero atom double and triple bonds, addition-elimination reactions, acid-base catalysis;
- Radical substitution: reaction of halogens and alkanes;
- Oxidations and reductions: switching between the different oxidation levels of common functional groups (alkyne – alkene – alkane – alkyl halide, alcohol – aldehyde, ketone – carboxylic acid derivatives, nitriles – carbonates)
- Cyclohexane conformations;
- Grignard reaction, Fehling and Tollens reaction;
- Simple polymers and their preparation (polystyrene, polyethylene, polyamides, polyesters);
- Amino acids and their classification in groups, isoelectric point, peptide bond, peptides and proteins;
- Carbohydrates: open chain and cyclic form of glucose and fructose;
- Lipids: general formulae of triacyl glycerides, saturated and unsaturated fatty acids;
进阶知识部分(适用于USNCO National Exam或更高级别,不适用中国赛)
• VSEPR theory in detail (with more than 4 ligands);
• Inorganic stereochemistry, isomerism in complexes;
• Solid state structures (metals, NaCl, CsCl) and Bragg’s law;
• Relation of equilibrium constants, electromotive force and standard Gibbs energy;
• Integrated rate law for first order reactions, half-life, Arrhenius equation,
determination of activation energy;
• Analysis of complex reactions using steady-state and quasi-equilibrium
approximations, mechanisms of catalytic reactions, determination of reaction order
and activation energy for complex reactions;
• Collision theory
• Simple phase diagrams and the Clausius-Clapeyron equation, triple and critical points;
• Stereoselective transformations (diastereoselective, enantioselective), optical purity
• Conformational analysis, use of Newman projections, anomeric effect
• Aromatic nucleophilic substitution, electrophilic substitution on polycyclic aromatic
compounds and heterocycles
• Supramolecular chemistry
• Advanced polymers, rubbers, copolymers, thermosetting polymers. Polymerization
types, stages and kinetics of polymerization;
• Amino acid side groups, reactions and separation of amino acids, protein sequencing;
• Secondary, tertiary and quaternary structures of proteins, non-covalent interactions,
stability and denaturation, protein purification by precipitation, chromatography and
electrophoresis;
• Enzymes and classification according to reaction types, active sites, coenzymes and
cofactors, mechanism of catalysis;
• Monosaccharides, equilibrium between linear and cyclic forms, pyranoses and
furanoses, Haworth projection and conformational formulae;
• Chemistry of carbohydrates, oligo and polysaccharides, glycosides, determination of
structure;
• Bases, nucleotides and nucleosides with formulae, Functional nucleotides, DNA and
RNA, hydrogen bonding between bases, replication, transcription and translation,
DNA based applications;
• Complex solubility calculations (with hydrolysing anions, complex formation);
• Simple Schrödinger equations and spectroscopic calculations;
• Simple MO theory;
• Basics of mass spectrometry (molecular ions, isotope distributions);
• Interpretation of simple NMR spectra (chemical shift, multiplicity, integrals);
• Synthesis techniques: filtrations, drying of precipitates, thin layer chromatography.
• Synthesis in microscale equipment,;
• Advanced inorganic qualitative analysis;
• Gravimetric analysis;
• Use of a spectrophotometer;
• Theory and practice of extraction with immiscible solvents;
• Column chromatography;
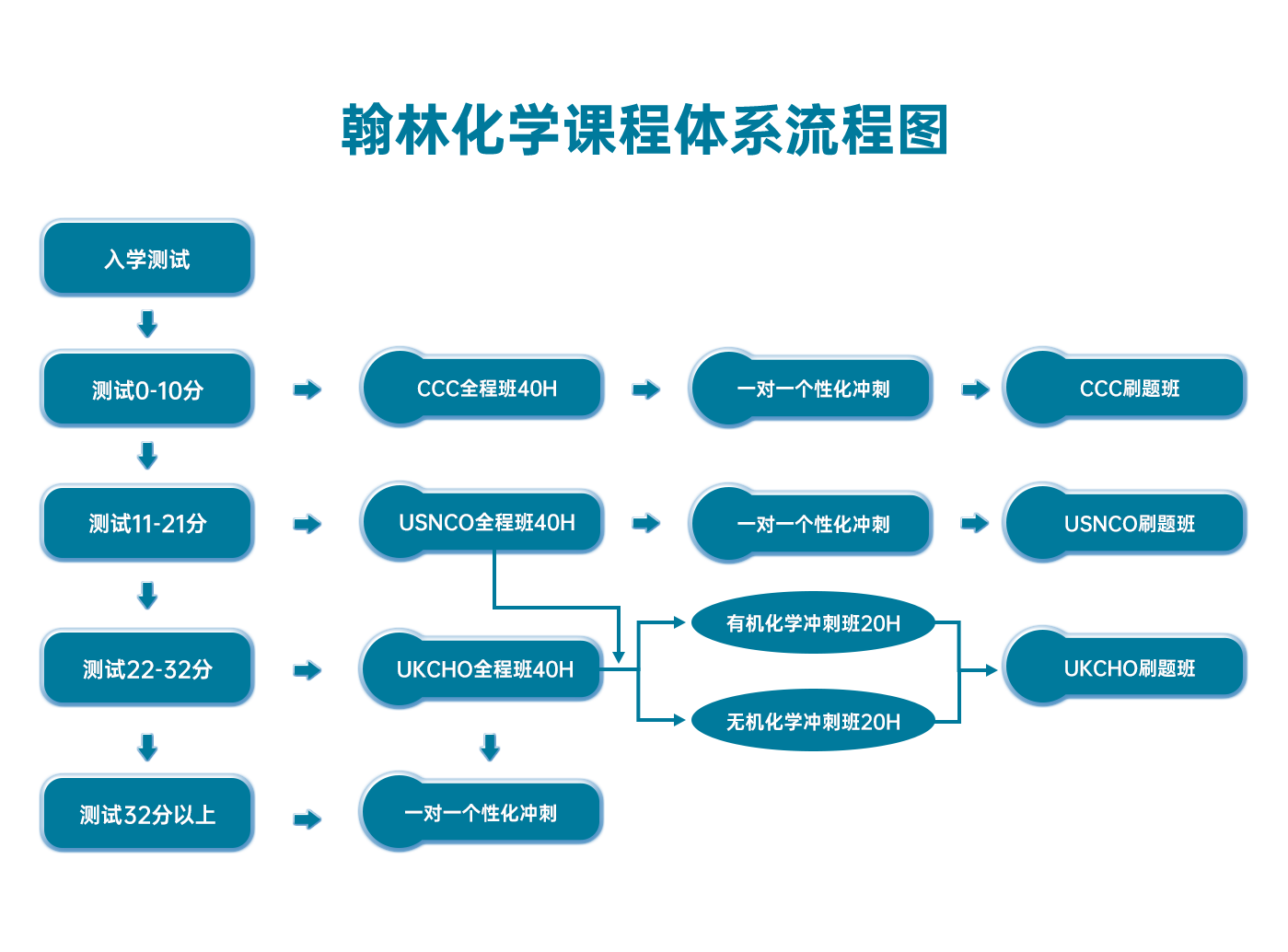
翰林化学学术活动课程体系流程图
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1






