- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记4.7.1 Density
Density
- Density is the mass per unit volume of an object
- Objects made from low-density materials typically have a lower mass
- For example, a balloon is less dense than a small bar of lead despite occupying a larger volume
- The units of density depend on the units used for mass and volume:
- If the mass is measured in g and volume in cm3, then the density will be in g / cm3
- If the mass is measured in kg and volume in m3, then the density will be in kg / m3

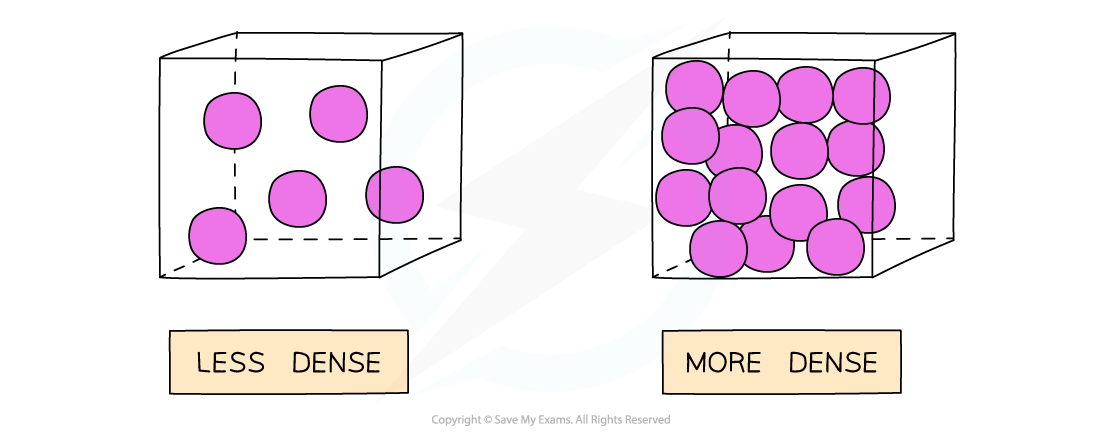
Gases are less dense than a solid
- The volume of an object may not always be given directly, but can be calculated with the appropriate equation depending on the object’s shape
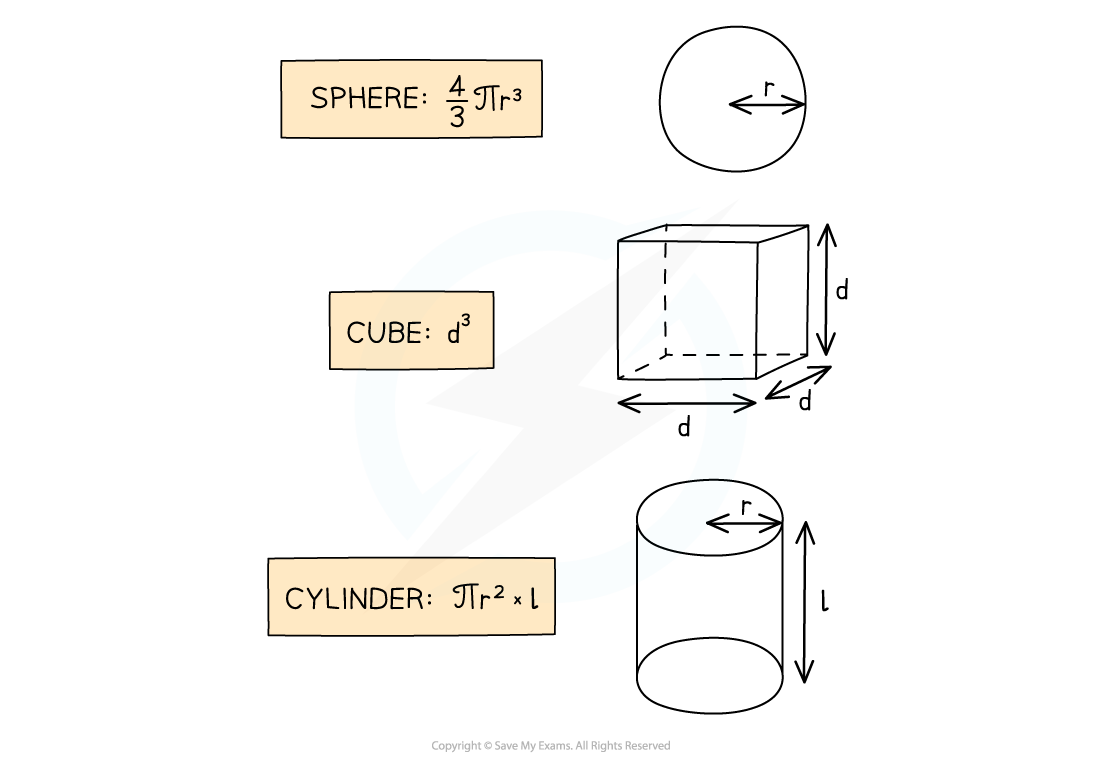
Volumes of common 3D shapes
Worked Example
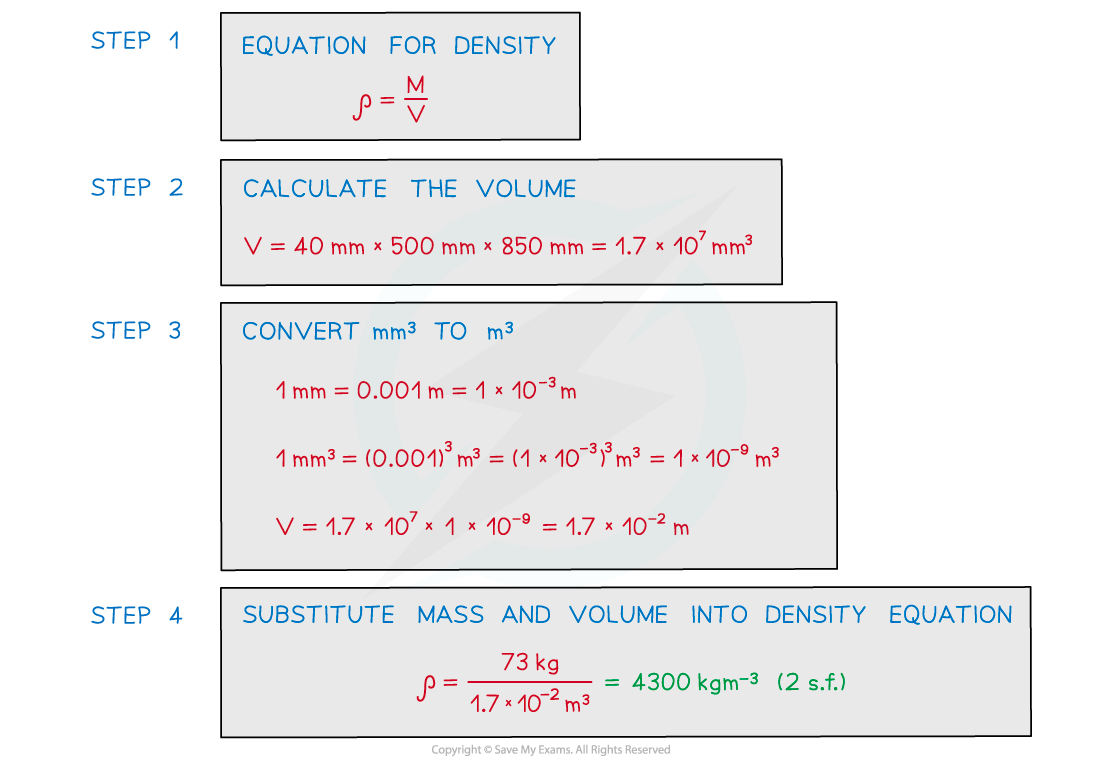
A paving slab has a mass of 73 kg and dimensions 40 mm × 500 mm × 850 mm.
Calculate the density, in kg m-3 of the material from which the paving slab is made.
Exam Tip
- When converting a larger unit to a smaller one, you multiply (×)
- E.g. 125 m = 125 × 100 = 12 500 cm
- When you convert a smaller unit to a larger one, you divide (÷)
- E.g. 5 g = 5 / 1000 = 0.005 or 5 × 10-3 kg
- When dealing with squared or cubic conversions, cube or square the conversion factor too
- E.g. 1 mm3 = 1 / (1000)3 = 1 × 10-9 m3
- E.g. 1 cm3 = 1 / (100)3 = 1 × 10-6 m3
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





