- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.4.2 Nitration of Benzene
Nitration
- The electrophilic substitution reaction in arenes consists of three steps:
- Generation of an electrophile
- Electrophilic attack
- Regenerating aromaticity
Mechanism of electrophilic substitution
- The nitration of benzene is one example of an electrophilic substitution reaction
- A hydrogen atom is replaced by a nitro (-NO2) group

The overall reaction of nitration of arenes
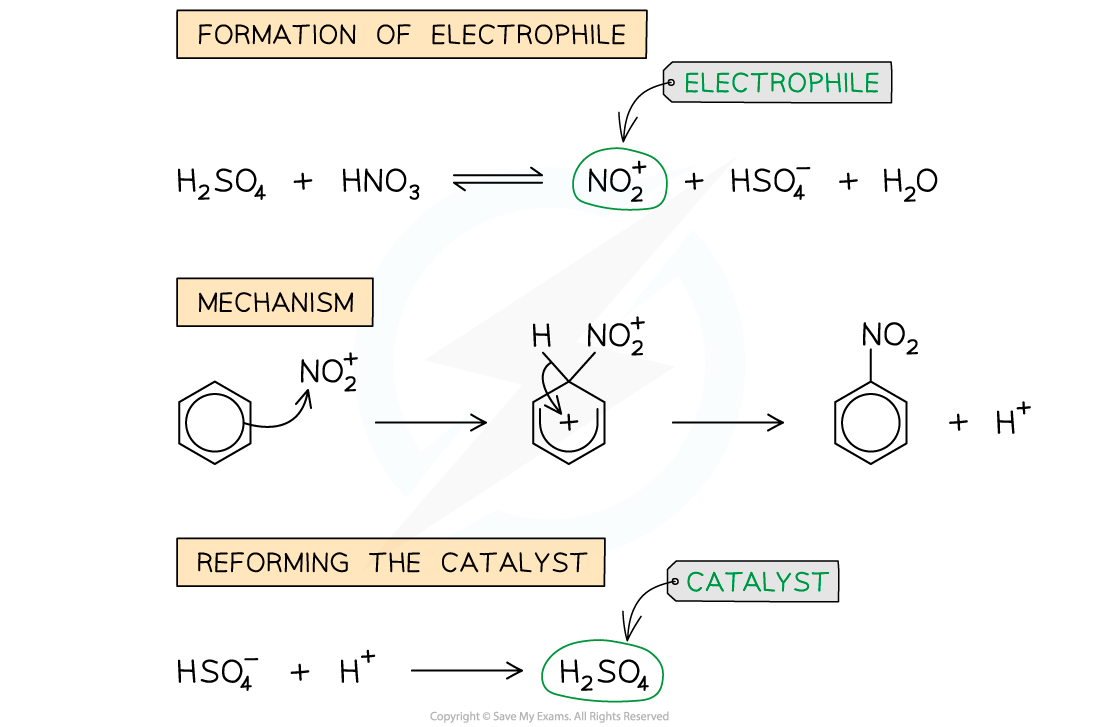
- In the first step, the electrophile is generated
- The electrophile NO2+ ion is generated by reacting concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4)
- Once the electrophile has been generated, it will carry out an electrophilic attack on the benzene ring
- The nitrating mixture of HNO3 and H2SO4 is refluxed with the arene at 25 - 60 oC
Nitration of Benzene Mechanism:
Addition reactions of arenes
- The delocalisation of electrons (also called aromatic stabilisation) in arenes is the main reason why arenes predominantly undergo substitution reactions over addition reactions
- In substitution reactions,
- In addition reactions, on the other hand, the aromaticity is not restored and is in some cases completely lost
- The hydrogenation of arenes is an example of an addition reaction during which the aromatic stabilisation of the arene is completely lost
- The cyclohexane formed is energetically less stable than the benzene
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





