- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.3.5 Acylation
Acyl Groups
Acyl groups
- Acyl groups can be built into many molecules using acyl chlorides or acid anhydrides (known as acylating agents)
- Acyl chlorides are derivatives of carboxylic acids by substitution of the -OH group by a chlorine atom
- Acyl chlorides are named by identifying the parent hydrocarbon chain and adding the suffix -oyl chloride
- They can also be named by removing the -oic acid from the carboxylic acid and adding -oyl chloride
- Acid anhydrides are also derivatives of carboxylic acids formed by substitution of the -OH group by an alkanoate
- Acid anhydrides are named by identifying the parent hydrocarbon chain and adding the suffix -oic anhydride
- They can also be named by removing the -oic acid from the carboxylic acid and adding -oic anyhydride
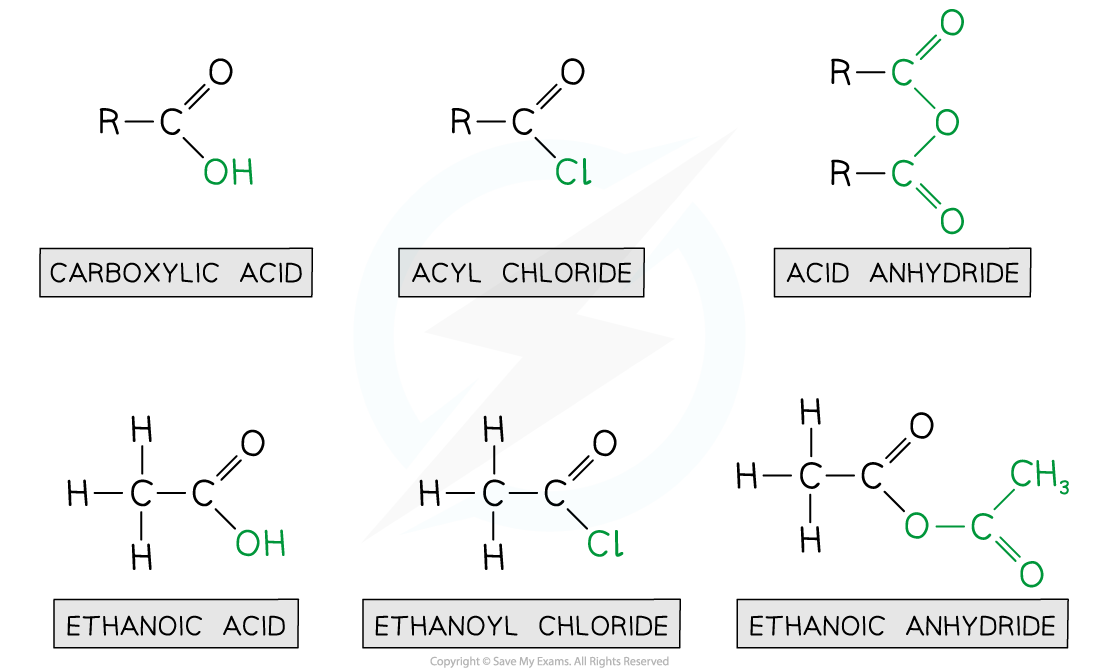
Ethanoic acid derivatives
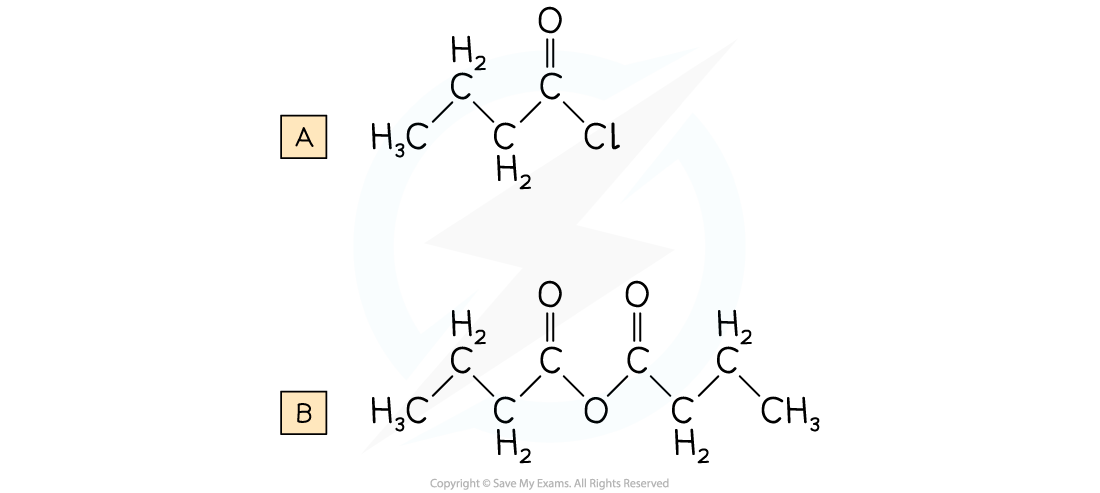
Worked Example
Draw the displayed formula for the following:a) butanoyl chlorideb) butanoic anhydride
Answer:
Nucleophilic Addition–Elimination
- Acyl chlorides are reactive organic compounds that undergo many reactions such as nucleophilic addition-elimination reactions
- In nucleophilic addition-elimination reactions, the nucleophilic addition of a small molecule across the C=O bond takes place followed by elimination of a small molecule
- Examples of these nucleophilic addition-elimination reactions include:
- Hydrolysis
- Reaction with alcohols to form esters
- Reaction with ammonia and primary amines to form amides
Hydrolysis
- The hydrolysis of acyl chlorides results in the formation of a carboxylic acid and HCl molecule
- This is a nucleophilic addition-elimination reaction
- A water molecule adds across the C=O bond
- A hydrochloric acid (HCl) molecule is eliminated
- An example is the hydrolysis of propanoyl chloride to form propanoic acid and HCl

Acyl chlorides are hydrolysed to carboxylic acids
Formation of esters
- Acyl chlorides can react with alcohols to form esters
- The esterification of acyl chlorides is also a nucleophilic addition-elimination reaction
- The alcohol adds across the C=O bond
- A HCl molecule is eliminated

Acyl chlorides undergo esterification with alcohols to form esters
Formation of amides
- Acyl chlorides can form amides with primary amines and ammonia
- The nitrogen atom in ammonia and primary amine has a lone pair of electrons which can be used to attack the carbonyl carbon atom in the acyl chlorides
- The product is an amide (when reacted with ammonia) or N-substituted amide (when reacted with primary amines)
- This is also an example of a nucleophilic addition-elimination reaction as
- The amine or ammonia molecule adds across the C=O bond
- A HCl molecule is eliminated
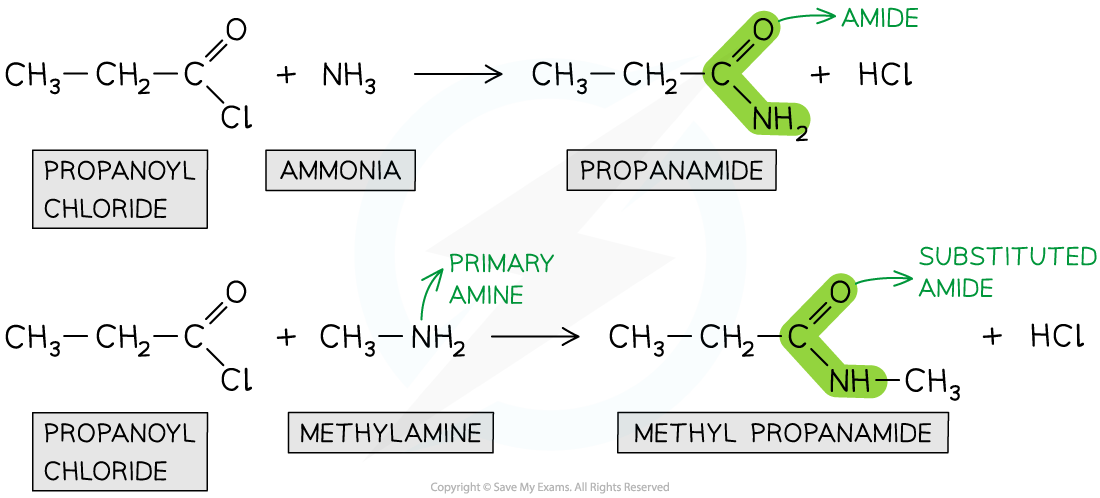
Acyl chlorides undergo reactions with ammonia and primary amines to form amides
Nucleophilic Addition–Elimination Mechanism
- Acyl chlorides undergo nucleophilic addition-elimination reactions such as hydrolysis, esterification reactions to form esters, and condensation reactions to form amides
- The general mechanism of these nucleophilic addition-elimination reactions involve two steps:
- Step 1 - Addition of a nucleophile across the C=O bond
- Step 2 - Elimination of a small molecule such as HCl or H2O
Mechanism of hydrolysis of acyl chlorides
- In the hydrolysis of acyl chlorides, the water molecule acts as a nucleophile
- Step 1 - Nucleophilic addition; the lone pair on the oxygen atoms carry out an initial attack on the carbonyl carbon
- Step 2 - Elimination; this is followed by the elimination of a hydrochloric acid (HCl) molecule
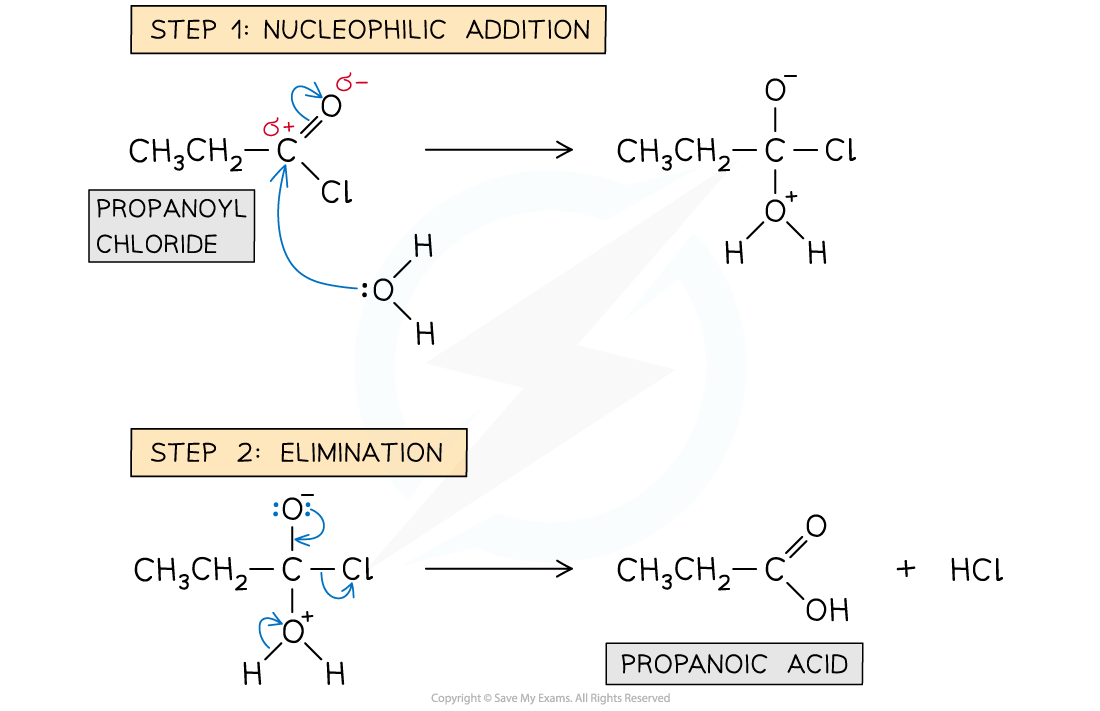
Reaction mechanism of the hydrolysis of acyl chlorides
Formation of esters: reaction mechanism
- In the esterification reaction of acyl chlorides, the alcohols act as a nucleophile
- Step 1 - Nucleophilic addition; the lone pair on the oxygen atoms carry out an initial attack on the carbonyl carbon
- Step 2 - Elimination; this is again followed by the elimination of an HCl molecule
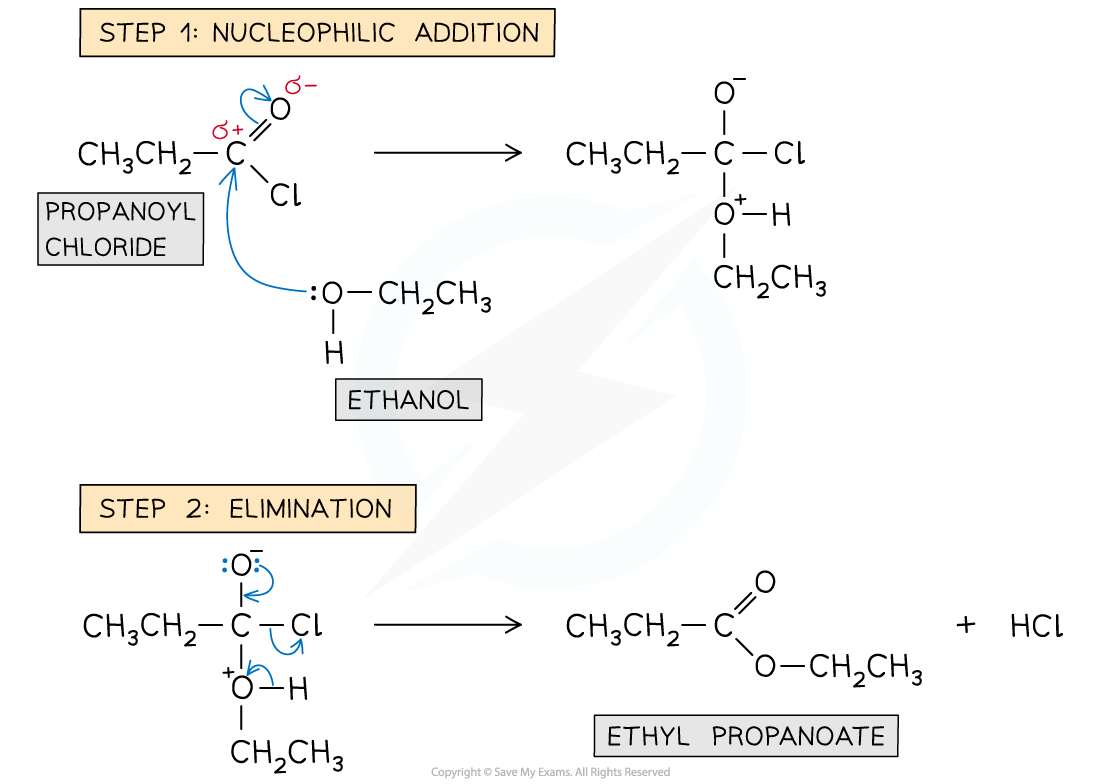
Reaction mechanism of the esterification of acyl chlorides with alcohols
Formation of amides: reaction mechanism
- The nitrogen atom in ammonia and primary amines act as a nucleophile
- Step 1 - Nucleophilic addition; the lone pair on the nitrogen atoms carry out an initial attack on the carbonyl carbon
- Step 2 - Elimination; this is followed by the elimination of an HCl molecule
- Step 3 - Acid-Base reaction; the HCl formed would immediately react with excess ammonia to give ammonium chloride in an acid-base reaction
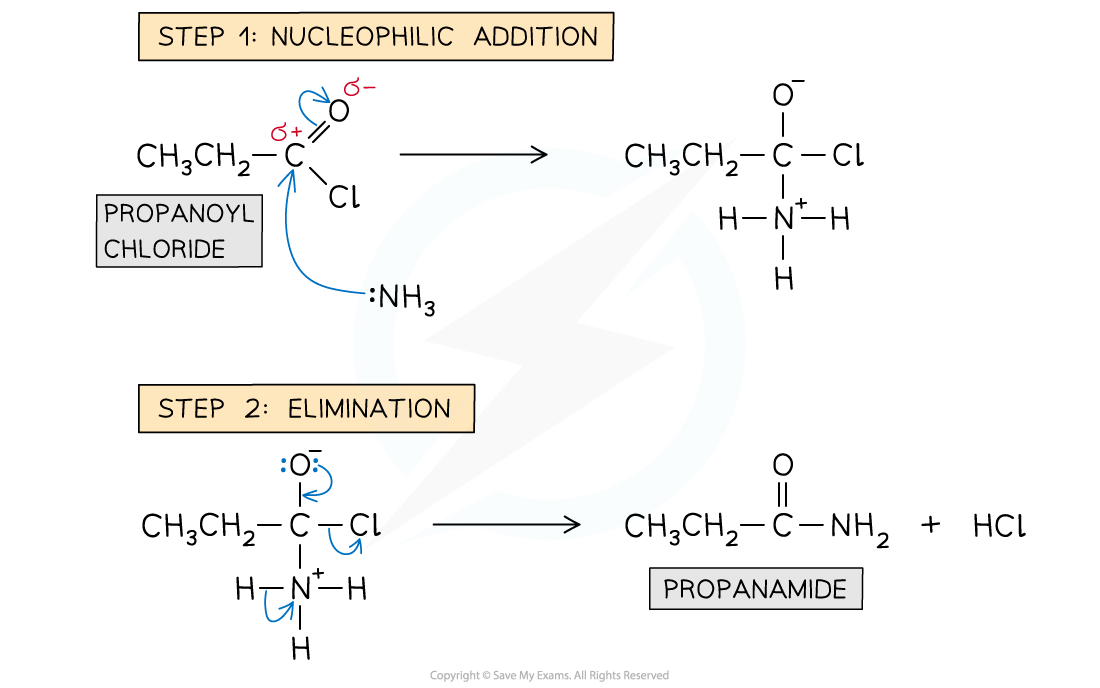
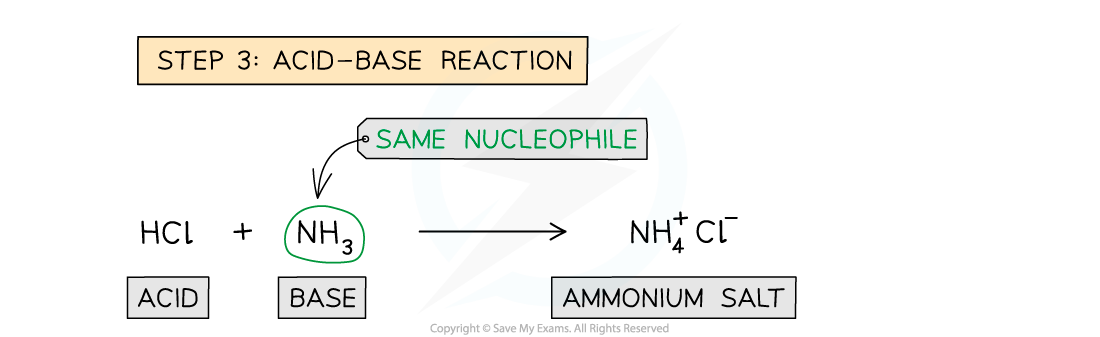
Reaction mechanism of the formation of amides from acyl chlorides with ammonia
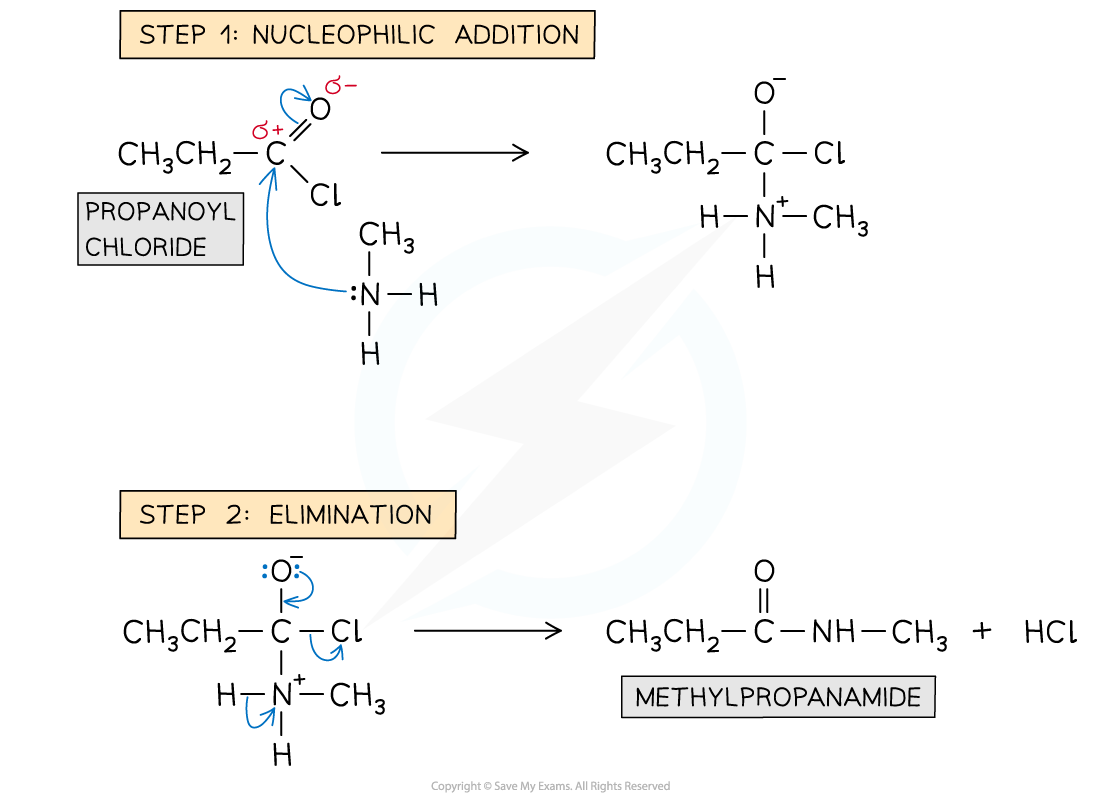
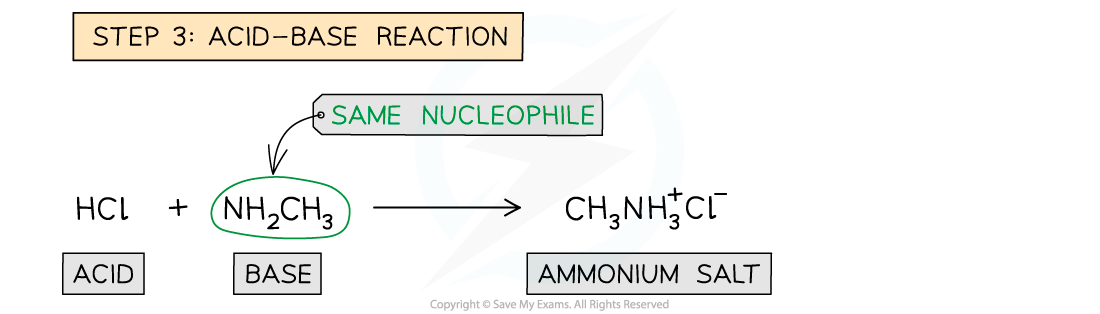
Reaction mechanism of the formation of amides from acyl chlorides with primary amines
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





