- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记6.3.2 Reactions with Bases
Reactions with Bases
- The differences in the the chemistry of +2 and +3 aqua ions can be seen in their reactions with bases
- The reactions of iron(II), iron(III), copper(II) and aluminium with bases is summarised below
The reactions of metal-aqua ions with bases table
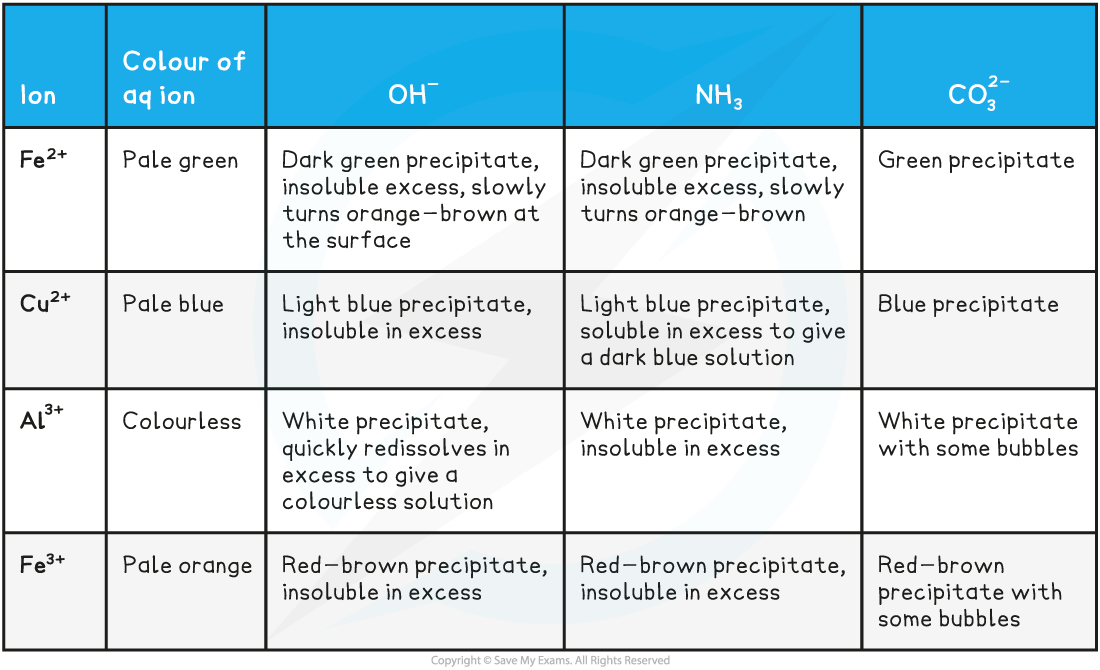
Iron(II)
- The dark green precipitate formed by reaction with hydroxide ions and with ammonia is hydrated iron(II) hydroxide
- This is formed in a two-step process
[Fe(H2O)6] 2+ (aq) + OH– (aq) → [Fe(H2O)5(OH)] + (aq) + H2O (l)
[Fe(H2O)5(OH)] + (aq) + OH– (aq) → Fe(H2O)4(OH)2 (s) + H2O (l)
- The ammonia behaves in the same way as sodium hydroxide as it is a base and removes protons from the water ligands; the overall reaction with ammonia is:
[Fe(H2O)6] 2+ (aq) + 2NH3 (aq) → Fe(H2O)4(OH)2 (s) + 2NH4+ (aq)
- With carbonate ions, the iron(II)carbonate precipitates out:
[Fe(H2O)6] 2+ (aq) + CO32- (aq) → FeCO3 (s) + 6H2O (l)
Copper(II)
- The light blue precipitate formed by reaction with hydroxide ions is hydrated copper(II) hydroxide
- This is formed in a two-step process
[Cu(H2O)6] 2+ (aq) + OH– (aq) → [Cu(H2O)5(OH)] + (aq) + H2O (l)
[Cu(H2O)5(OH)] + (aq) + OH– (aq) → Cu(H2O)4(OH)2 (s) + H2O (l)
- The ammonia initially behaves in the same way as sodium hydroxide as it is a base and removes protons from the water ligands
[Cu(H2O)6] 2+ (aq) + 2NH3 (aq) → Cu(H2O)4(OH)2 (s) + 2NH4+ (aq)
- However, ammonia is a good ligand and in excess ammonia, the ammonia partially substitutes for water creating the deep blue complex ion, dihydroxytetraaminecopper(II)
Cu(H2O)4(OH)2 (s) + 4NH3 (aq) → [Cu(NH3)4(H2O)2 ]2+ (aq) + + 2OH- (aq) + 2H2O (l)
- With carbonate ions, the copper(II)carbonate precipitates out:
[Cu(H2O)6] 2+ (aq) + CO32- (aq) → CuCO3 (s) + 6H2O (l)
Aluminium ions
- The white precipitate formed by reaction with hydroxide ions and with ammonia is hydrated aluminium hydroxide
- This is formed in a three-step process
[Al(H2O)6] 3+ (aq) + OH– (aq) → [Al(H2O)5(OH)] 2+ (aq) + H2O (l)
[Al(H2O)5(OH)] 2+ (aq) + OH– (aq) → [Al(H2O)4(OH)2] + (aq) + H2O (l)
[Al(H2O)4(OH)2] + (aq) + OH– (aq) → Al(H2O)3(OH)3 (s) + H2O (l)
- The ammonia behaves in the same way as sodium hydroxide as it is a base and removes protons from the water ligands; the overall reaction with ammonia is:
[Al(H2O)6] 3+ (aq) + 3NH3 (aq) → Al(H2O)3(OH)3 (s) + 3NH4+ (aq)
- With carbonate ions, the reaction is a little more complicated
- In the previous section we saw that +3 ions are acidic in water, so the addition of carbonate ions liberates bubbles of carbon dioxide:
2H3O+ (aq) + CO32- (aq) → CO2 (g) + 3H2O (l)
- The reaction between aluminium and water exists in an equilibrium:
[Al(H2O)6] 3+ (aq) + 3H2O (l) ⇌ Al(H2O)3(OH)3 (s) + 3H3O+ (aq)
- Removal of the hydronium ions by carbonate ions pushes the equilibrium to the right and precipitates out the hydrated aluminium hydroxide
- The overall equation can therefore shown as:
2[Al(H2O)6]3+ + 3CO32− (aq) → 2Al(H2O)3(OH)3 (s) + 3CO2 (g) + 3H2O (l)
Iron(III)
- The red-brown precipitate formed by reaction with hydroxide ions and with ammonia is hydrated iron(III) hydroxide
- This is formed in a three-step process
[Fe(H2O)6] 3+ (aq) + OH– (aq) → [Fe(H2O)5(OH)] 2+ (aq) + H2O (l)
[Fe(H2O)5(OH)] 2+ (aq) + OH– (aq) → [Fe(H2O)4(OH)2] + (aq) + H2O (l)
[Fe(H2O)4(OH)2] + (aq) + OH– (aq) → Fe(H2O)3(OH)3 (s) + H2O (l)
- The ammonia behaves in the same way as sodium hydroxide as it is a base and removes protons from the water ligands; the overall reaction with ammonia is:
[Fe(H2O)6] 3+ (aq) + 3NH3 (aq) → Fe(H2O)3(OH)3 (s) + 3NH4+ (aq)
- With carbonate ions, the reaction is the same as with aluminium; the acidity of iron(III) ions removes the carbonate ion and produces bubbles of carbon dioxide, while the iron(III) precipitates out as the hydroxide
[Fe(H2O)6] 3+ (aq) + 3H2O (l) ⇌ Fe(H2O)3(OH)3 (s) + 3H3O+ (aq)
- The overall equation can therefore shown as:
2[Fe(H2O)6]3+ (aq) + 3CO32− (aq) → 2Fe(H2O)3(OH)3 (s) + 3CO2 (g) + 3H2O (l)
Exam Tip
Transition metals in the +3 state are acidic and do not form carbonate precipitates, unlike the +2 ions.
Amphoteric Hydroxides
- Aluminium hydroxide is classified as an amphoteric hydroxide
- The word amphoteric means it reacts with both acids and bases
- Aluminium hydroxide is insoluble in water but readily dissolves in dilute hydrochloric acid producing the hexaaquaaluminium ion:
Al(OH)3(H2O)3 (s) + 3HCl (aq) → [Al(H2O)6] 3+ (aq) + 3Cl- (aq)
- Aluminium hydroxide dissolves in sodium hydroxide to form sodium tetrahydoxoaluminate
Al(OH)3(H2O)3 (s) + NaOH (aq) → Na [Al(OH)4] (aq) + 3H2O (l)
- The same equation may be shown ionically as:
Al(OH)3(H2O)3 (s) + OH- (aq) → [Al(OH)4] - (aq) + 3H2O (l)
- You need a strong base to carry out the reaction, so it is usually done with hot concentrated sodium hydroxide
Exam Tip
You can also show the reactions with sodium hydroxide as:Al(OH)3 + NaOH → NaAl(OH)4orAl(OH)3(H2O)3 + OH– → [Al(OH)4(H2O)2]– + H2OorAl(OH)3 + OH– → [Al(OH)4]–
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





