- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记 6.2.3 Shapes of Complex Ions
Shapes of Complex Ions
- Depending on the size of the ligands and the number of dative bonds to the central metal ion, transition element complexes have different geometries
- Dative bonds can also be referred to as coordinate bonds, especially when discussing the geometry of a complex
Linear
- Central metal atoms or ions with two coordinate bonds form linear complexes
- The bond angles in these complexes are 180o
- The most common examples are a copper (I) ion, (Cu+), or a silver (I) ion, (Ag+), as the central metal ion with two coordinate bonds formed to two ammonia ligands

Examples of a linear complex
- The second example is the diamminesilver(I) ion, [Ag(NH₃)₂]⁺, which is present in Tollens' reagent
- Tollens' reagent is used to test for the aldehyde functional group in organic molecules
- In the test, the silver(I) ion is reduced to silver atoms that produce a characteristic silver mirror on the test tube walls
Tetrahedral
- When there are four coordinate bonds the complexes often have a tetrahedral shape
- Complexes with four chloride ions most commonly adopt this geometry
- Chloride ligands are large, so only four will fit around the central metal ion
- The bond angles in tetrahedral complexes are 109.5o

Example of a tetrahedral complex
Square planar
- Sometimes, complexes with four coordinate bonds may adopt a square planar geometry instead of a tetrahedral one
- Cyanide ions (CN-) are the most common ligands to adopt this geometry
- An example of a square planar complex is cisplatin
- The bond angles in a square planar complex are 90o

Cisplatin is an example of a square planar complex
Octahedral
- Octahedral complexes are formed when a central metal atom or ion forms six coordinate bonds
- This could be six coordinate bonds with six small, monodentate ligands
- Examples of such ligands are water and ammonia molecules and hydroxide and thiocyanate ions
- It could be six coordinate bonds with three bidentate ligands
- Each bidentate ligand will form two coordinate bonds, meaning six coordinate bonds in total
- Examples of these ligands are 1,2-diaminoethane and the ethanedioate ion
- It could be six coordinate bonds with one multidentate ligand
- The multidentate ligand, for example EDTA4-, forms all six coordinate bonds
- The bond angles in an octahedral complex are 90o
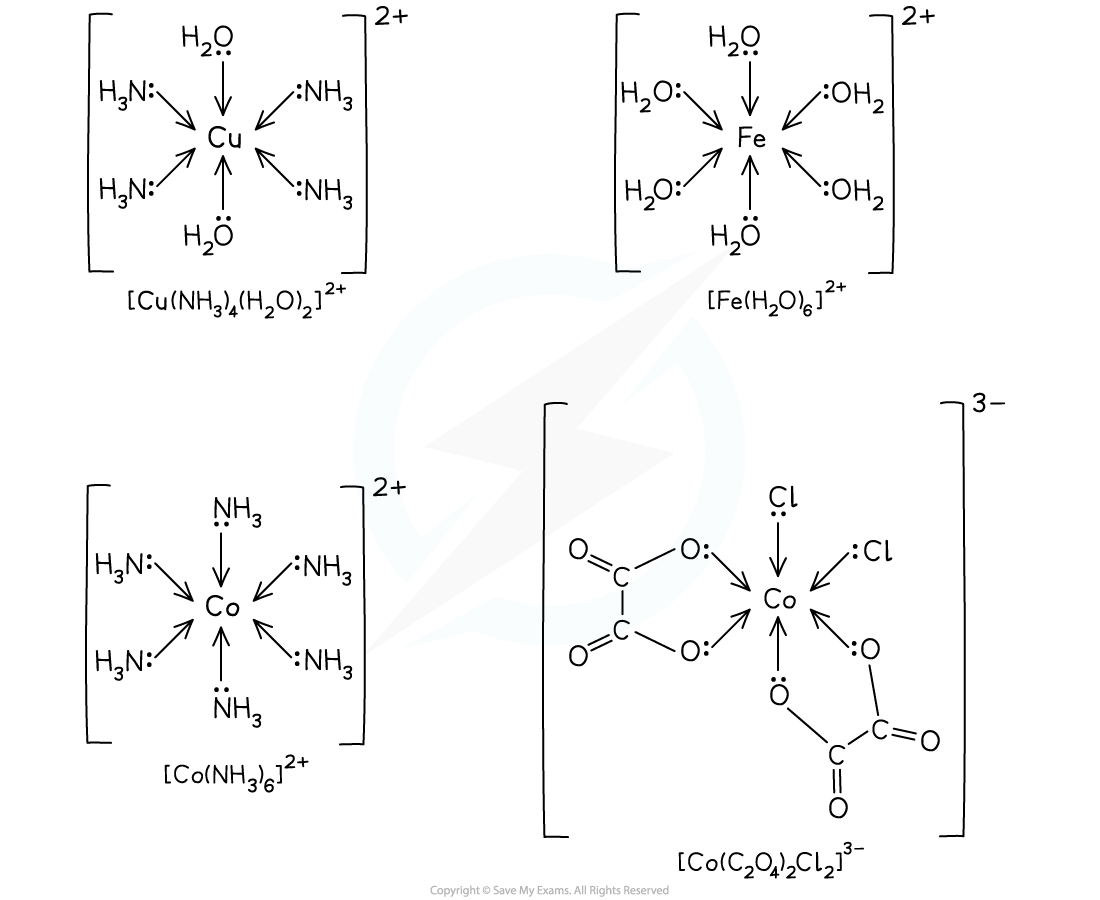
Examples of octahedral complexes
Isomerism in Complex Ions
- Transition element complexes can exhibit stereoisomerism
Geometrical (cis-trans) isomerism
- Even though transition element complexes do not have a double bond, they can still have geometrical isomers
- Square planar and octahedral complexes with two pairs of different ligands exhibit cis-trans isomerism (this is a special case of E-Z isomerism)
- An example of a square planar complex with two pairs of ligands is the anti-cancer drug cis-platin
- Whereas cis-platin has beneficial medical effects by binding to DNA in cancer cells, trans-platin cannot be used in cancer treatment

Cis-platin (the Z-isomer) and trans-platin (the E-isomer) is an example of a square planar transition element complex that exhibits geometrical isomerism
- As long as a complex ion has two ligands attached to it that are different to the rest, then the complex can display geometric isomerism
- Examples of octahedral complexes that exhibit geometrical isomerism are the [Cu(NH3)4(H2O)2]2+ and [Ni(H2NCH2CH2NH2)2Cl2]2+ complexes
- [Ni(H2NCH2CH2NH2)2Cl2]2+ can also be written as [Ni(en)2Cl2]2+
- Like in the square planar complexes, if the two ‘different’ ligands are adjacent (next) to each other then that is the ‘cis’ isomer, and if the two ‘different’ ligands are opposite each other then this is the ‘trans’ isomer
- In [Cu(NH3)4(H2O)2]2+, the two water ligands are adjacent to each other in the cis isomer and are opposite each other in the trans isomer
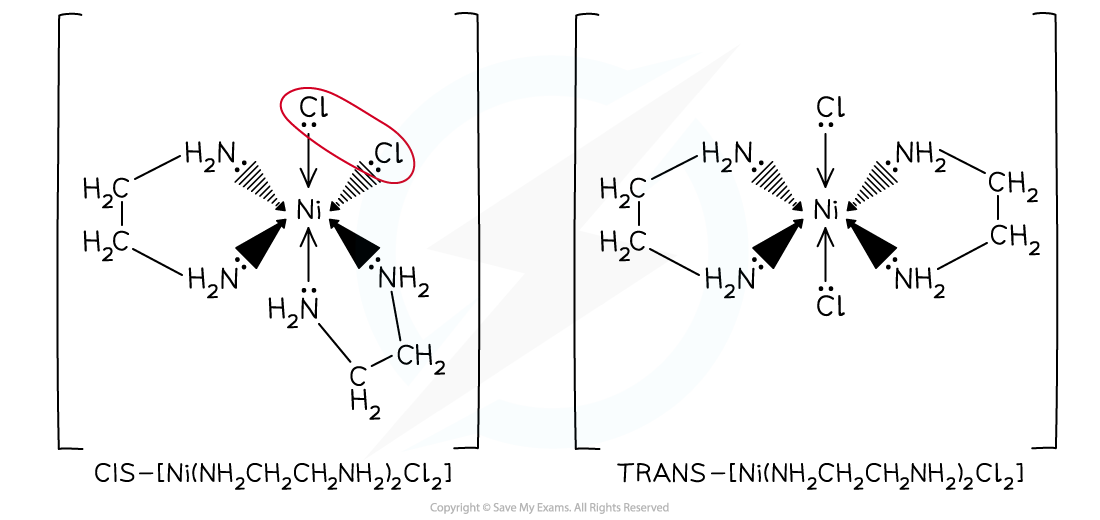

Octahedral transition metal complexes exhibiting geometrical isomerism
Optical isomerism
- Octahedral complexes with bidentate ligands also have optical isomers
- This means that the two forms are non-superimposable mirror images of each other
- They have no plane of symmetry, and one image cannot be placed directly on top of the other
- The optical isomers only differ in their ability to rotate the plane of polarised light in opposite directions
- Examples of octahedral complexes that have optical isomers are the [Ni(H2NCH2CH2NH2)3]2+and [Ni(H2NCH2CH2NH2)2(H2O)2]2+ complexes
- The ligand H2NCH2CH2NH2 can also be written as ‘en’ instead
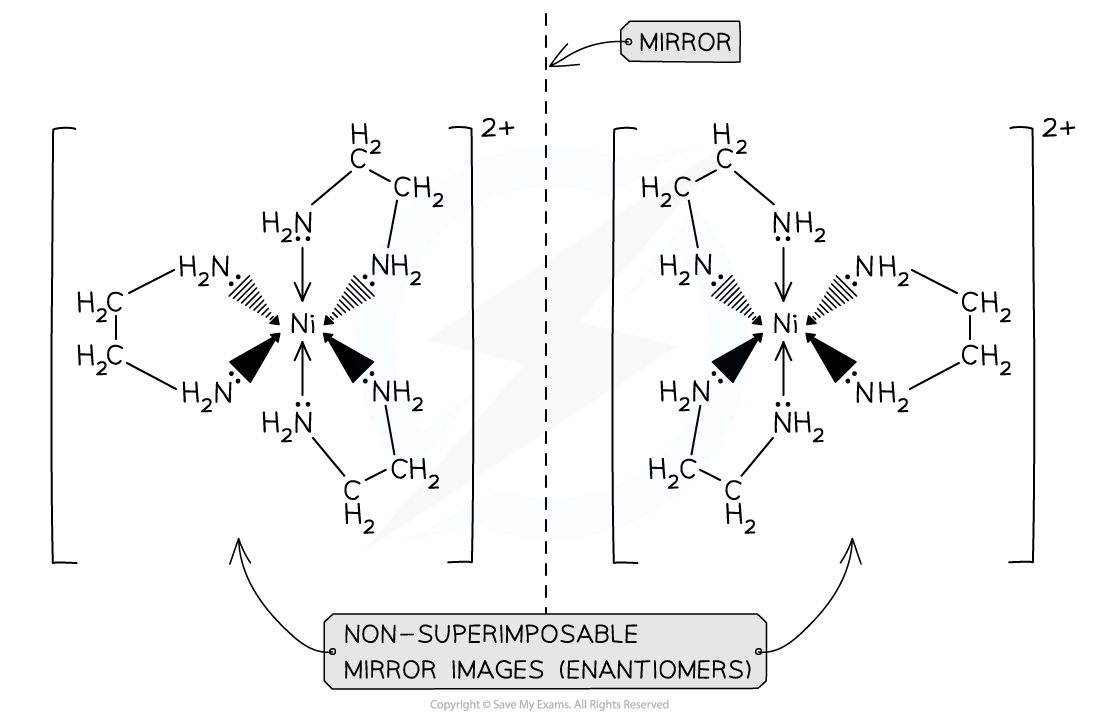
Octahedral transition metal complexes exhibiting optical isomerism
Drawing stereochemical formulae
- Chemists use a convention of wedge drawings to represent three dimensional molecules
- The convention is that
- a solid line is a bond in the same plane as the paper
- a dotted line is a bond receding behind the plane of the paper(this can also be hatched or shaded wedges)
- a solid wedge is a bond coming out of the paper
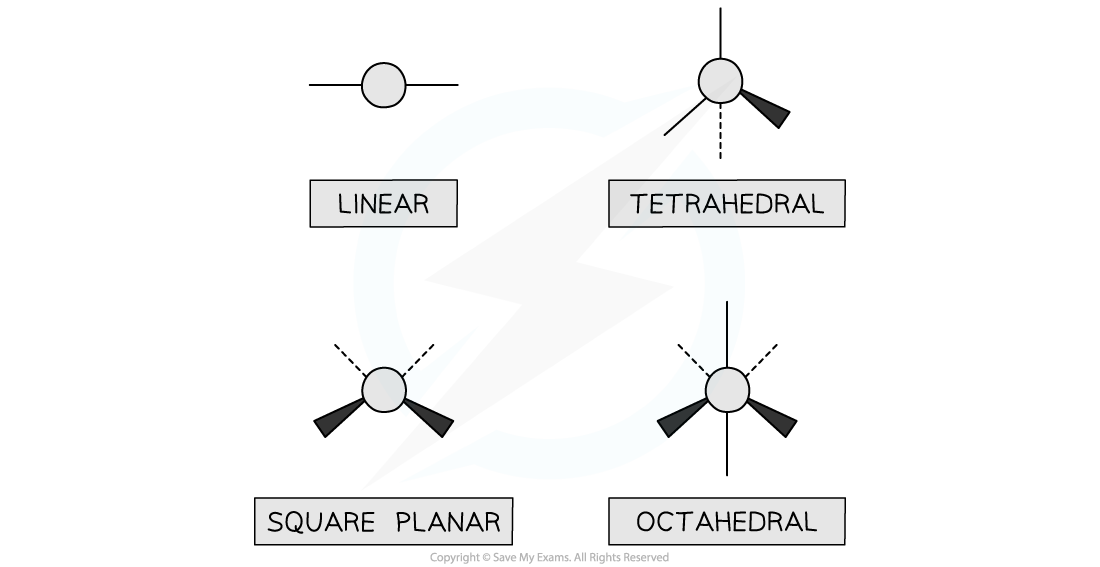
Four main shapes of transition metal complexes using stereochemical formulae
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





