- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.7.5 Electrophilic Addition
Mechanism: Electrophilic Addition
Electrophilic addition
- Alkenes undergo electrophilic addition reactions
- In an electrophilic addition reaction, two reactants form only one product
- So, electrophilic addition reactions will have a 100% atom economy
- It is the double bond in an alkene which makes them so reactive
- The C=C double bond is an electron-rich area of the molecule which is readily attacked by positively charged electrophiles
- Alkenes will undergo electrophilic addition reactions with hydrogen halides, halogens and concentrated sulfuric acid with steam
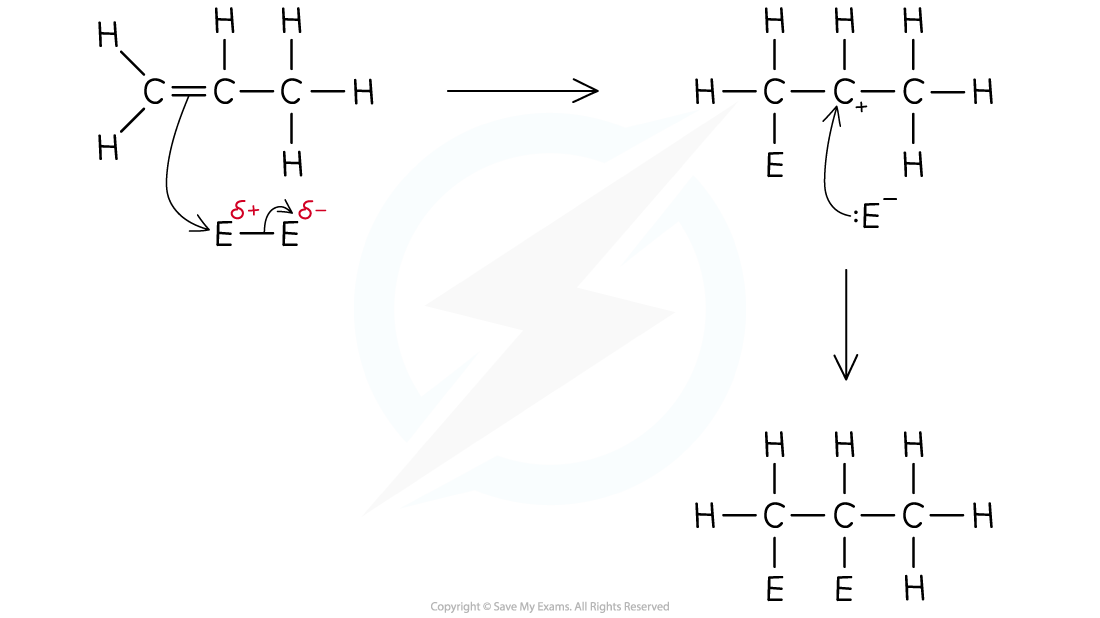
General mechanism for Electrophilic Addition
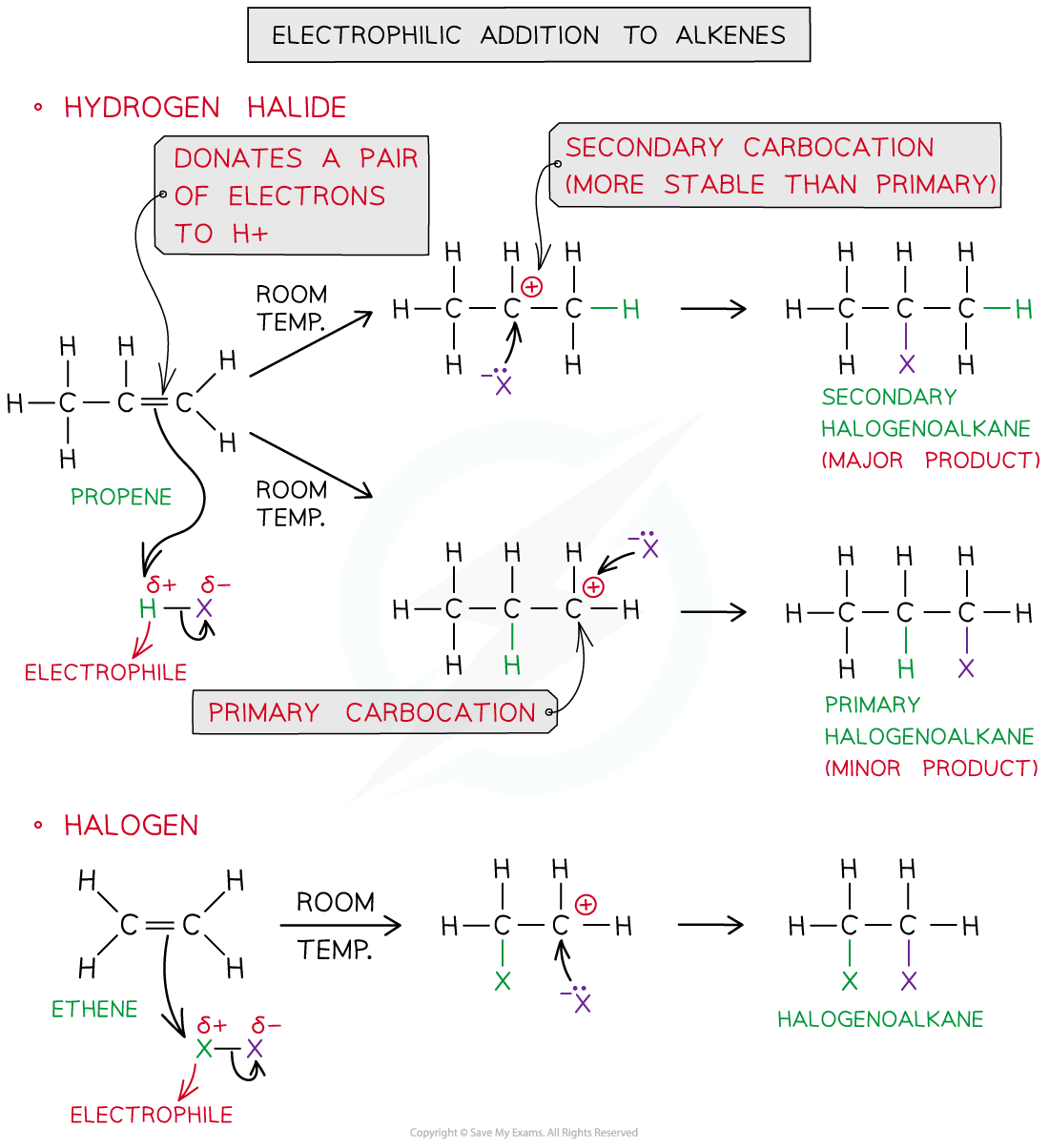
Electrophilic addition of hydrogen halides or halogens at room temperatures to alkenes results in the formation of halogenoalkanes
- When the reaction takes place with an asymmetrical alkene, then you need to determine which product will form
- This depends on the stability of the carbocation formed as the intermediate
- The stability of carbon carbocations is as follows:
- Tertiary > secondary > primary
- The major product formed will be from the intermediate with the more stable carbocation, but some of the product from the less stable carbocation intermediate will also form
- In the mechanism above, the secondary halogenoalkane is the major product, because a secondary carbocation is more stable than a primary carbocation
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





