- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记2.3.3 Testing for Halide Ions
Testing for Halides
Silver ions & ammonia
- Halide ions can be identified in an unknown solution by dissolving the solution in nitric acid and then adding silver nitrate solution dropwise
- The nitric acid is to prevent any false positive results from carbonate ions precipitating out with silver ions
- The halide ions will react with the silver nitrate solution as follows:
Ag+ (aq) + X- (aq) → AgX (s)
(ionic equation)
Where X- is the halide ion
- The state symbols are key in this equation
- If the unknown solution contains halide ions, a precipitate of the silver halide will be formed (AgX)
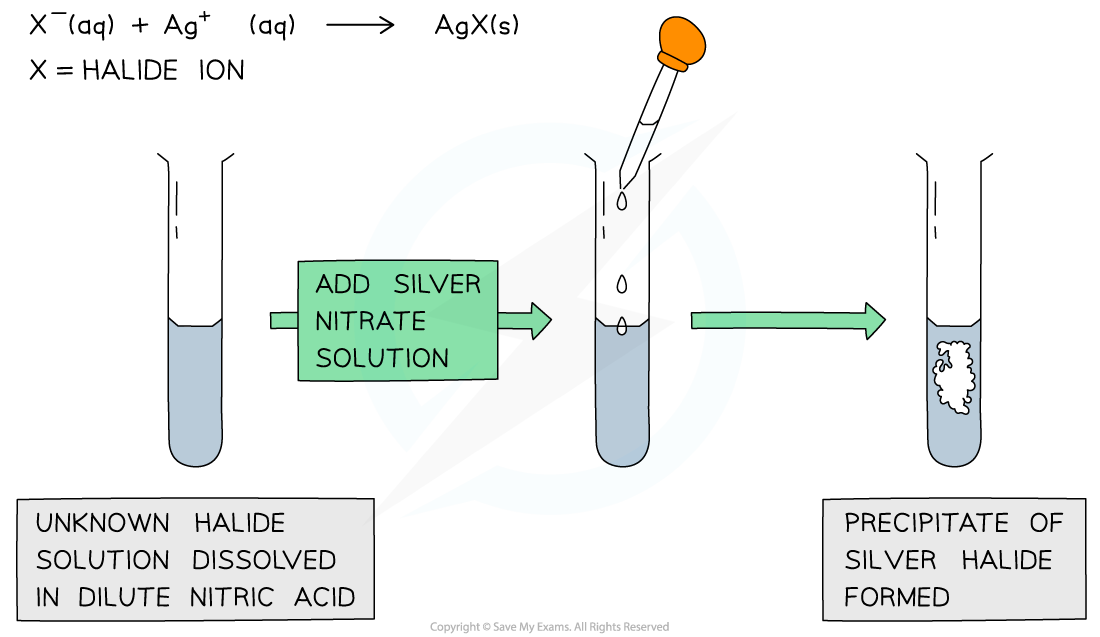
A silver halide precipitate is formed upon addition of silver nitrate solution to halide ion solution
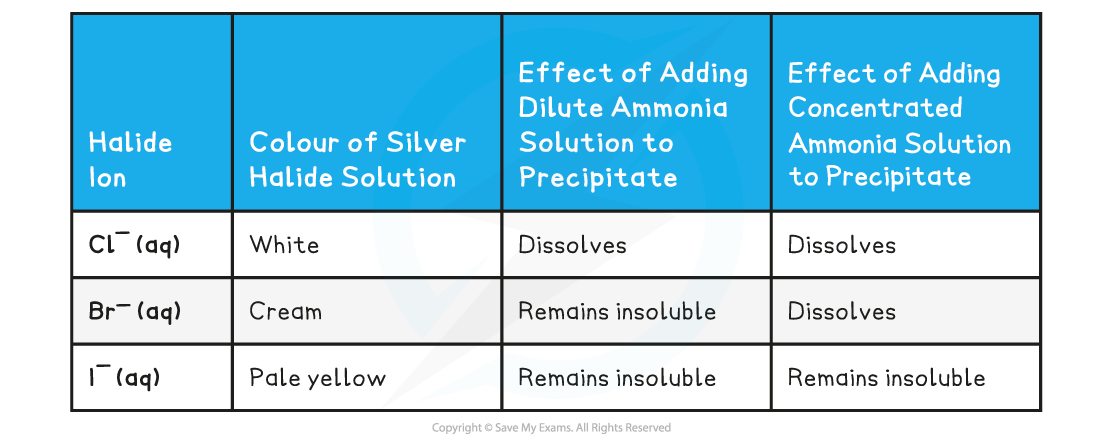
- Silver chloride (AgCl) is a white precipitate
- Silver bromide (AgBr) is a cream precipitate
- Silver iodide (AgI) is a yellow precipitate
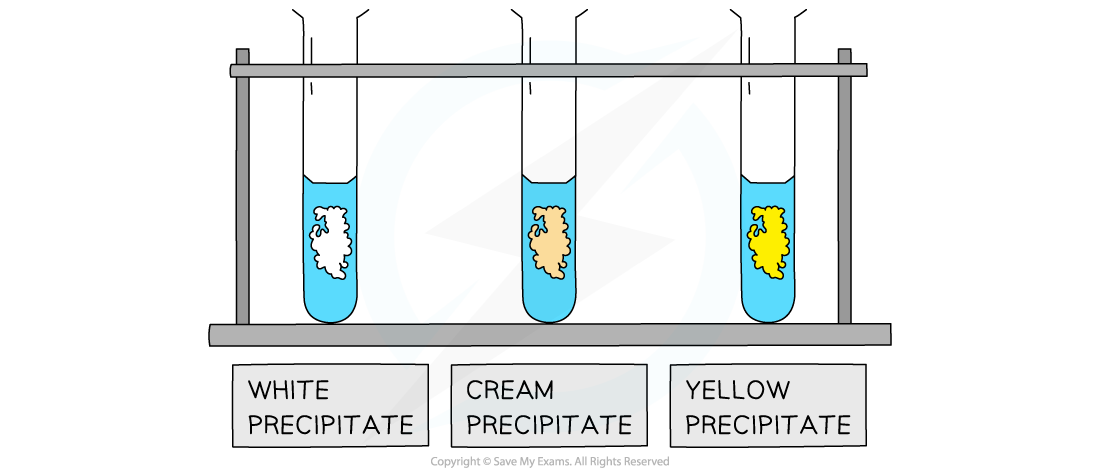
The silver halide precipitates are dense and characteristically coloured
Adding ammonia
- Because the white, cream and yellow precipitates could look very similar in colour, ammonia is often used as a follow up test to determine which halide ion is present
- Dilute followed by concentrated ammonia is added to the silver halide solution to identify the halide ion
- If the precipitate dissolves in dilute ammonia the unknown halide is chloride
- If the precipitate does not dissolve in dilute, but does dissolve in concentrated ammonia the unknown halide is bromide
- If the precipitate does not dissolve in dilute or concentrated ammonia, then the unknown halide is iodide
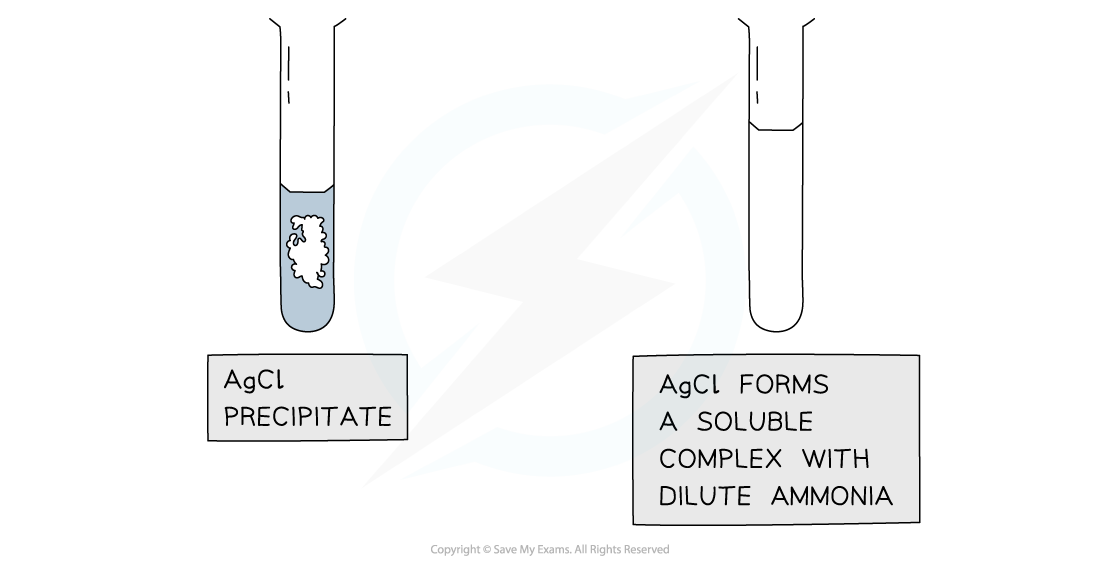
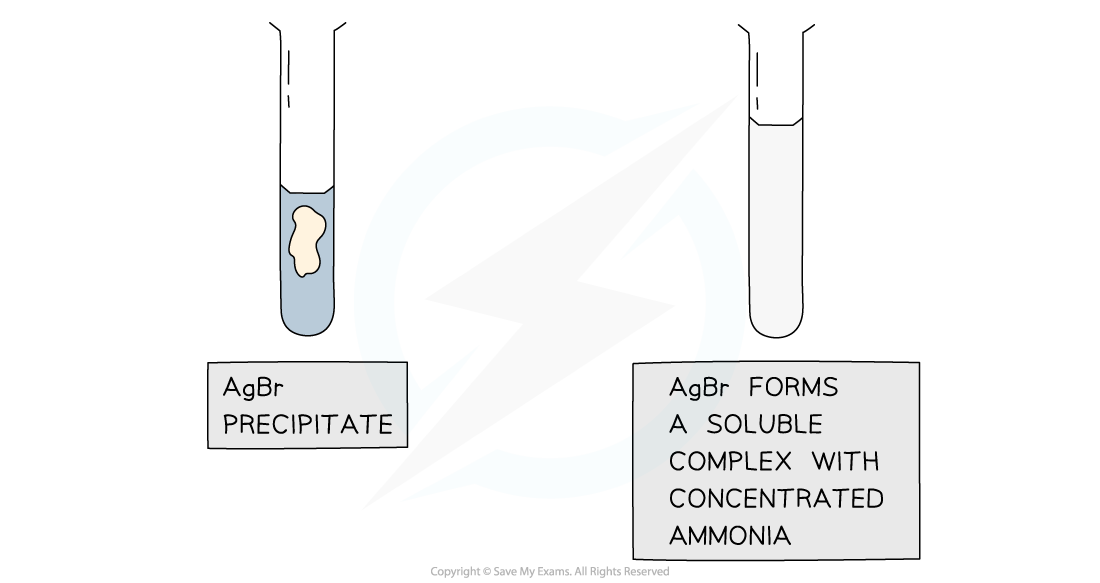
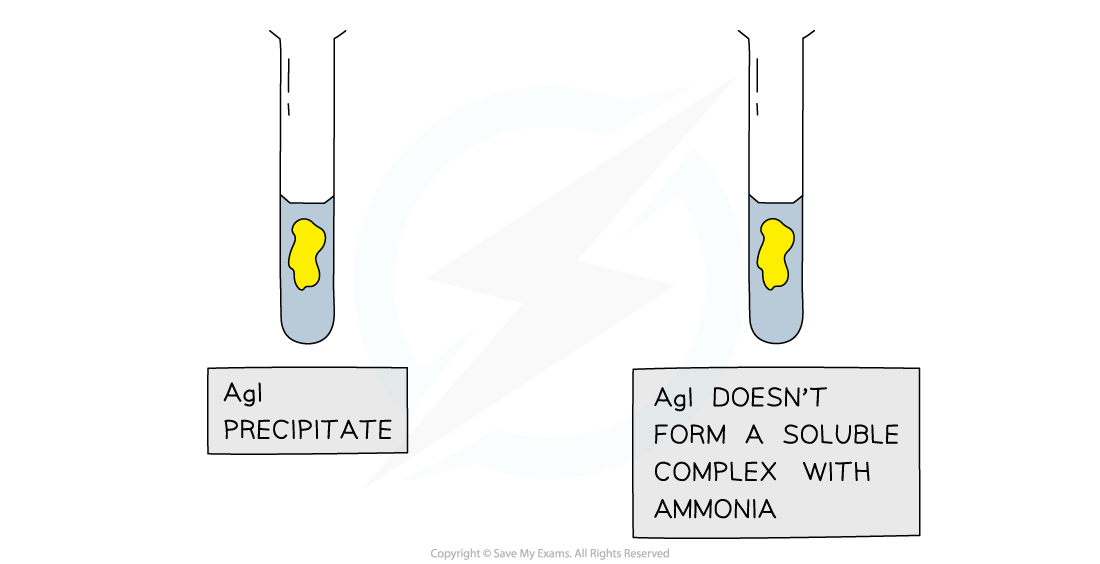
Silver chloride and silver bromide precipitates dissolve on addition of ammonia solution whereas silver iodide is insoluble in ammonia
Reaction of Halide Ions with Silver Nitrate & Ammonia Solutions
Concentrated sulfuric acid
- Chloride, bromide and iodide ions react with concentrated sulfuric acid to produce toxic gases
- These reactions should therefore be carried out in a fume cupboard
- The general reaction of the halide ions with concentrated sulfuric acid is:
H2SO4(l) + X-(aq) → HX(g) + HSO4-(aq)
(general equation)
where X is the halide ion
Reaction of chloride ions with concentrated sulfuric Acid
- Concentrated sulfuric acid is dropwise added to sodium chloride crystals to produce hydrogen chloride gas
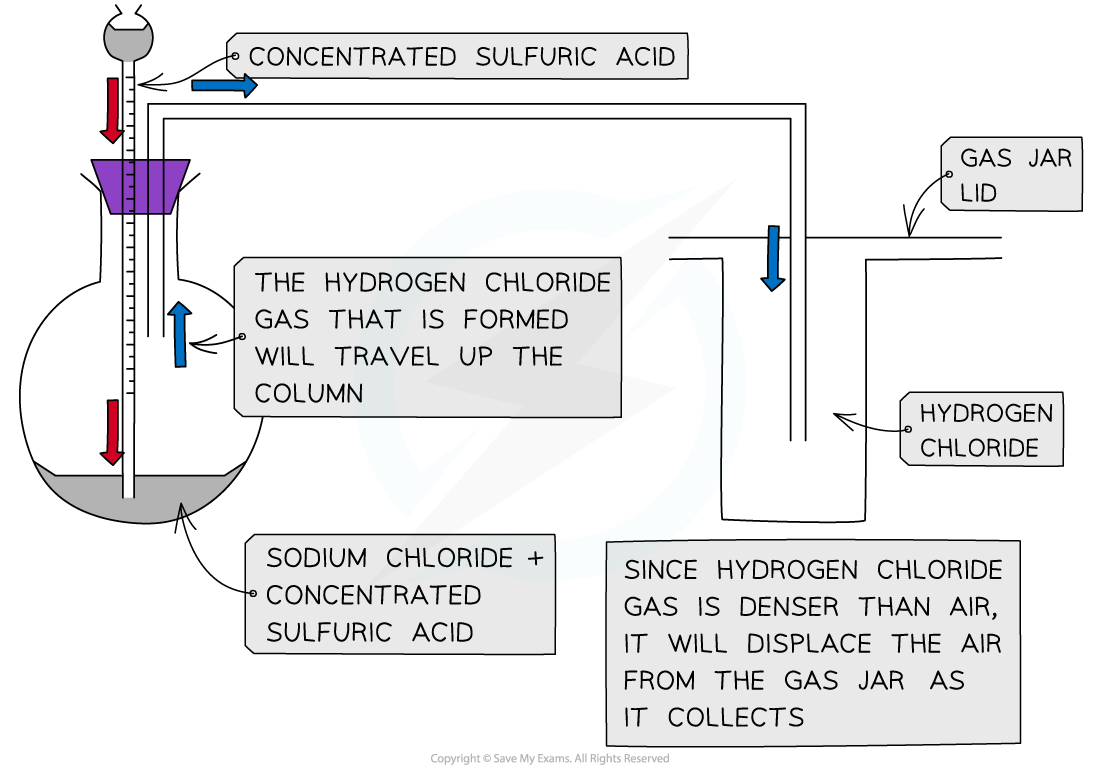
Apparatus set up for the preparation of hydrogen chloride gas from sodium chloride with concentrated sulfuric acid
- The reaction that takes place is:
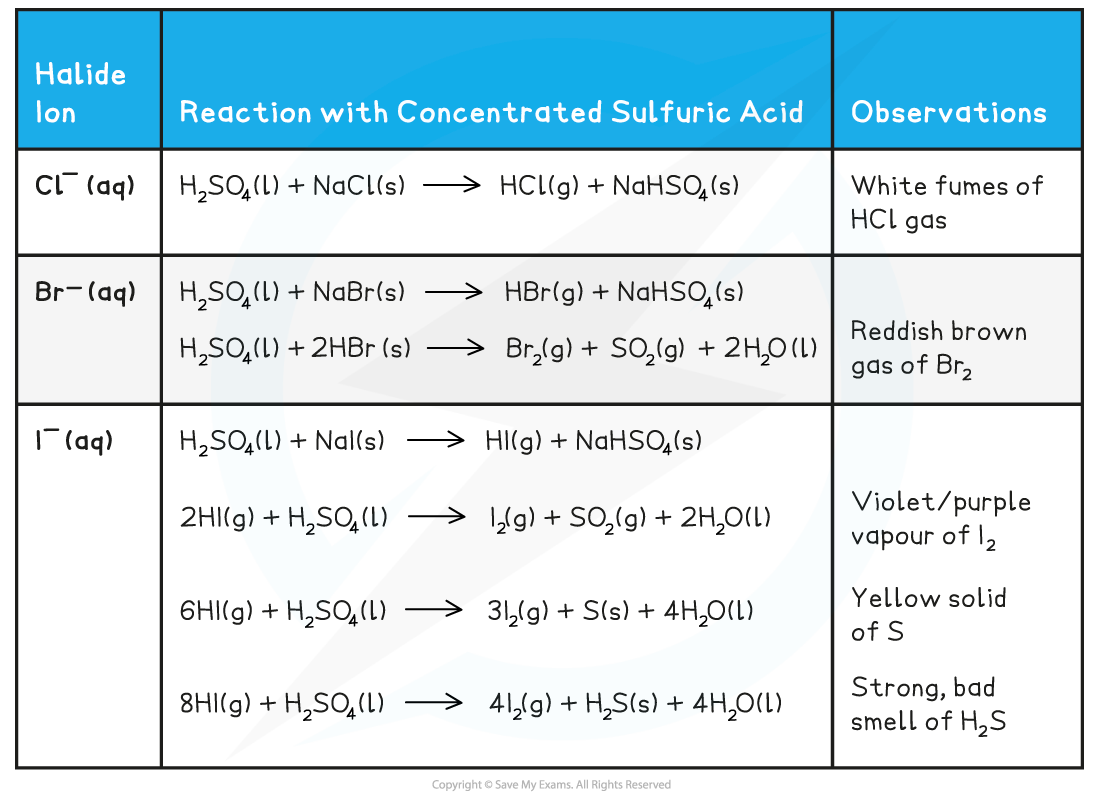
H2SO4 (l) + NaCl (s) → HCl (g) + NaHSO4 (s)
- The HCl gas produced is seen as white fumes
Reaction of bromide ions with concentrated sulfuric acid
- The reaction of sodium bromide and concentrated sulfuric acid is:
H2SO4 (l) + NaBr (s) → HBr (g) + NaHSO4 (s)
- The concentrated sulfuric acid oxidises HBr which decomposes into bromine and hydrogen gas and sulfuric acid itself is reduced to sulfur dioxide gas:
2HBr (g) + H2SO4 (l) → Br2 (g) + SO2 (g) + 2H2O (l)
- The bromine is seen as a reddish-brown gas
Reaction of iodide ions with concentrated sulfuric acid
- The reaction of sodium iodide and concentrated sulfuric acid is:
H2SO4 (l) + NaI (s) → HI (g) + NaHSO4 (s)
- Hydrogen iodide decomposes readily
- Sulfuric acid oxidises the hydrogen iodide to form several products:
- The concentrated sulfuric acid oxidises HI and is itself reduced to sulfur dioxide gas:
2HI (g) + H2SO4 (l) → I2 (g) + SO2 (g) + 2H2O (l)
- Iodine is seen as a violet/purple vapour
- The concentrated sulfuric acid oxidises HI and is itself reduced to sulfur:
6HI (g) + H2SO4 (l) → 3I2 (g) + S (s) + 4H2O (l)
- Sulfur is seen as a yellow solid
- The concentrated sulfuric acid oxidises HI and is itself reduced to hydrogen sulfide:
8HI (g) + H2SO4 (l) → 4I2 (g) + H2S (s) + 4H2O (l)
- Hydrogen sulfide has a strong smell of bad eggs
Summary of the Halide Ion Reactions with Concentrated Sulfuric Acid
Exam Tip
It gets easier to oxidise the hydrogen halides going down Group 7: the halides become stronger reducing agents.
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





