- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.9.3 Redox Equations
Balancing Redox Reactions
- Balancing equations using redox principles is a useful skill and is best illustrated by following an example
- It is important to follow a methodical step-by-step approach so that you don't get lost:
Worked Example
Writing overall redox reactions
Manganate(VII) ions (MnO4- ) react with Fe2+ ions in the presence of acid (H+) to form Mn2+ ions, Fe3+ ions and water
Write the overall redox equation for this reaction
Answer
Step 1: Write the unbalanced equation and identify the atoms which change in oxidation state

Step 2: Deduce the oxidation state changes
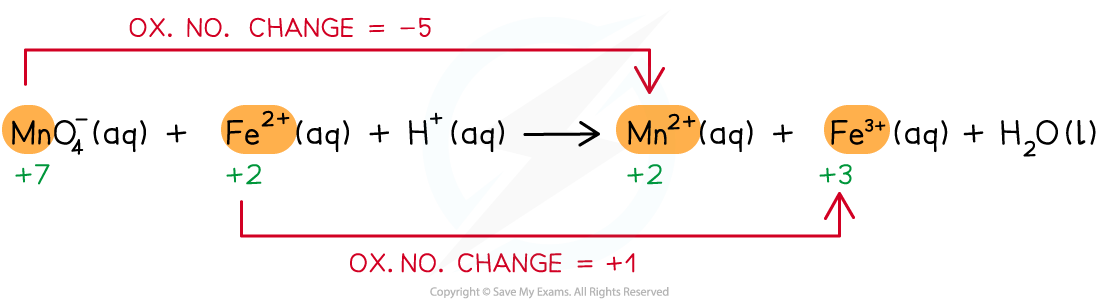
Step 3: Balance the oxidation state changes
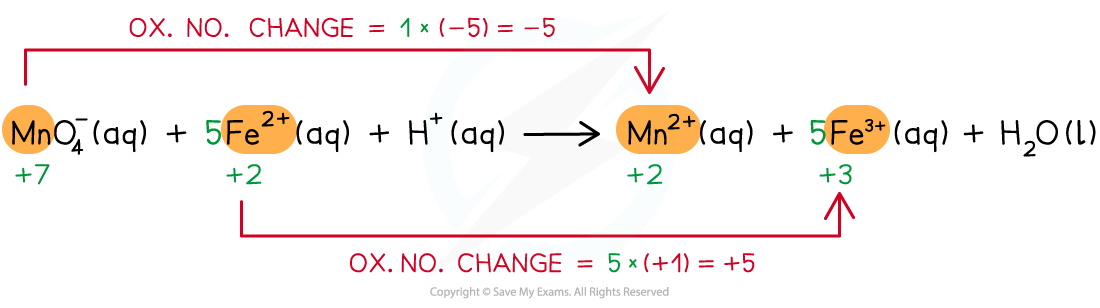
Step 4: Balance the charges
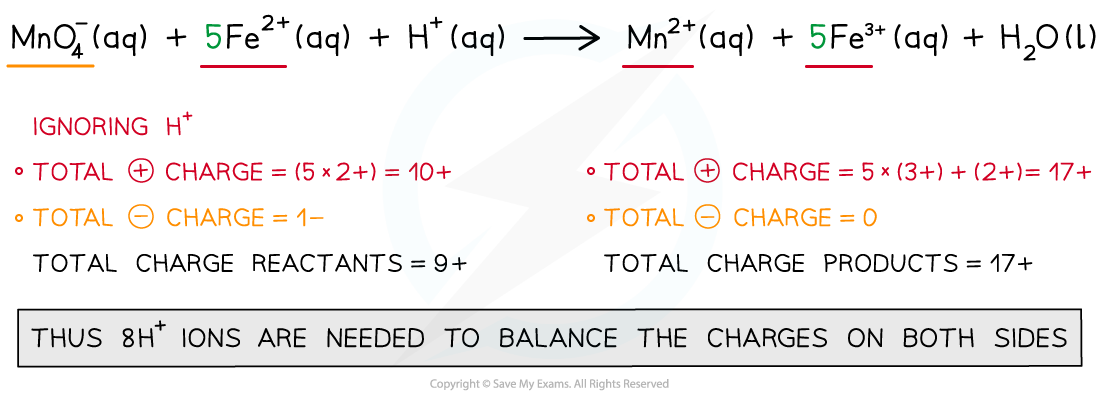
Step 5: Finally, balance the atoms

Redox & Disproportionation Reactions
Oxidation
- Oxidation is the gain of oxygen, eg:
Cu + H2O → CuO + H2
(Cu has gained an oxygen and is oxidised)
- Oxidation is also the loss of hydrogen, eg:
2NH3 + 3Br2 → N2 + 6HBr
(NH3 has lost hydrogen and is oxidised)
- Oxidation is also the loss of electrons, eg:
Cu2+ + Mg → Mg2+ + Cu
(Mg has lost two electrons and is oxidised)
- Oxidation causes an increase in oxidaiton state, eg:
Cu2+ + Mg → Mg2+ + Cu
(change in ox. no. of Mg is +2 thus Mg is oxidised)
Reduction
- Reduction is the loss of oxygen, eg:
Cu+ H2O → 2CuO + H2
(H2O has been reduced)
- Reduction is also the gain of hydrogen, eg:
2NH3+ 3Br2 → N2 + 6HBr
(Br has been reduced)
- Reduction is also the gain of electrons, eg:
Cu2+ + Mg → Mg2+ + Cu
(Cu has been reduced)
- Reduction causes a decrease in oxidation number, eg:
Cu2+ + Mg → Mg2+ + Cu
(the change in oxidation state of Cu is -2 thus Cu is reduced)
Redox reactions
- Redox reactions are reactions in which oxidation and reduction take place simultaneously
- While one species is oxidising, another is reducing in the same reaction, eg:
Cu2++ Mg → Mg2+ + Cu
(Cu has been reduced and Mg has been oxidised)
Worked Example
Oxidation and reductionIn each of the following equations, state which reactant has been oxidised and which has been reduced.
- Na++ Cl- → NaCl
- Mg + Fe2+ → Mg2+ + Fe
- CO + Ag2O → 2Ag + CO2
Answer
Answer 1:
-
- Oxidised: Cl- as the oxidation state has increased by 1
- Reduced: Na+ as the oxidation state has decreased by 1
Answer 2:
-
- Oxidised: Mg as the oxidation state has increased by 2
- Reduced: Fe2+ as the oxidation state has decreased by 2
Answer 3:
-
- Oxidised: C as it has gained oxygen
- Reduced: Ag as it has lost oxygen
Disproportionation reactions
- A disproportionation reaction is a reaction in which the same species is both oxidised and reduced
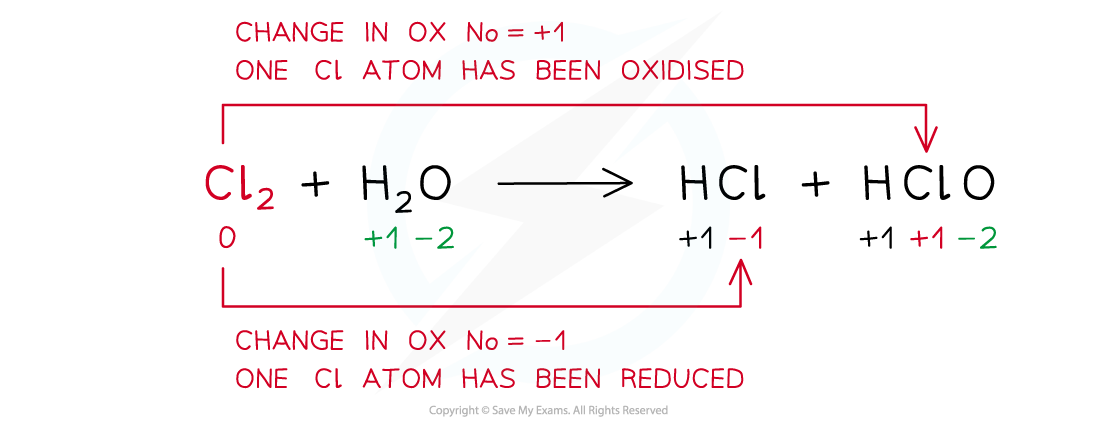
Example of a disproportion reaction in which the same species (chlorine in this case) has been both oxidised and reduced
Worked Example
Balancing disproportionation reactionsBalance the disproportionation reaction which takes place when chlorine is added to hot concentrated aqueous sodium hydroxideThe products are Cl- and ClO3- ions and water
Answer
Step 1: Write the unbalanced equation and identify the atoms that change in oxidation state:

Step 2: Deduce the oxidation state changes:
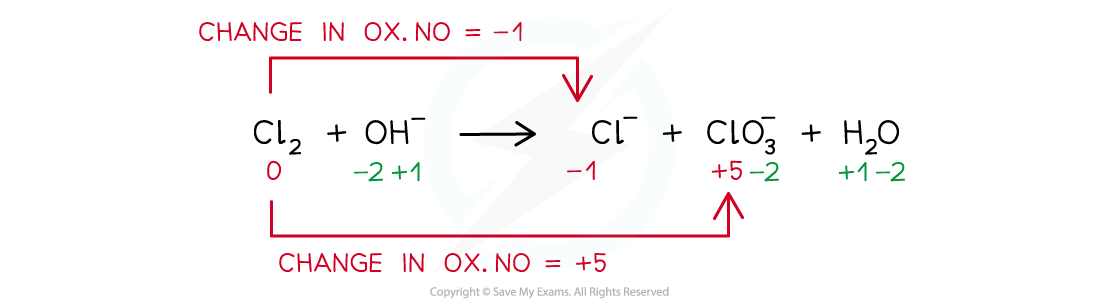
Step 3: Balance the oxidation state changes:
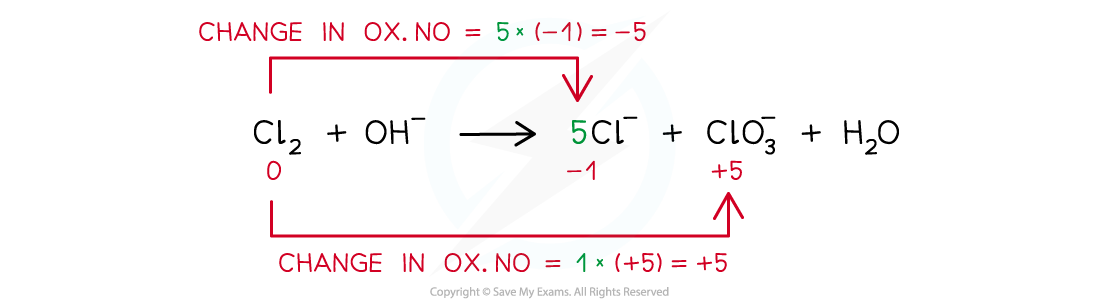
Step 4: Balance the charges

Step 5: Balance the atoms

转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





