- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.6.2 Energy Level Diagrams
Energy Level Diagrams
- An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction as the reaction proceeds ( called 'extent of the reaction' below)
- The transition state is a stage during the reaction at which chemical bonds are partially broken and formed
- The transition state is very unstable – a molecule in the transition state cannot be isolated and is higher in energy than the reactants and products
- The activation energy (Ea) is the energy needed to reach the transition state
- We can define the activation energy as ‘the minimum amount of energy needed for reactant molecules to have a successful collision and start the reaction’
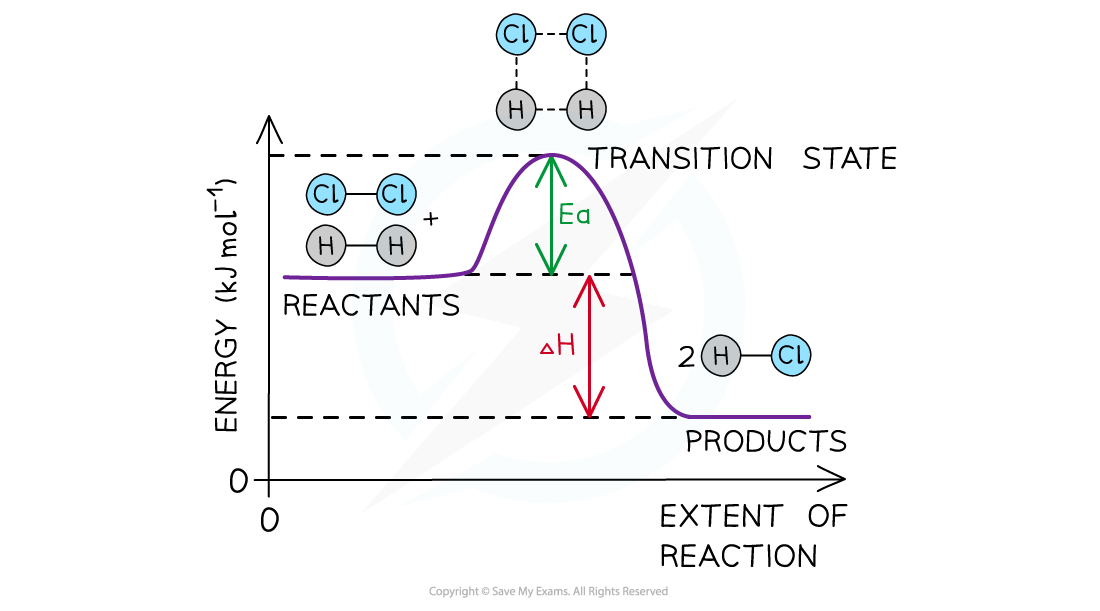
The energy level diagram for the reaction of hydrogen with chlorine to form hydrogen chloride gas
Exothermic reaction
- In an exothermic reaction, the reactants are higher in energy than the products
- The reactants are therefore closer in energy to the transition state
- This means that exothermic reactions have a lower activation energy compared to endothermic reactions
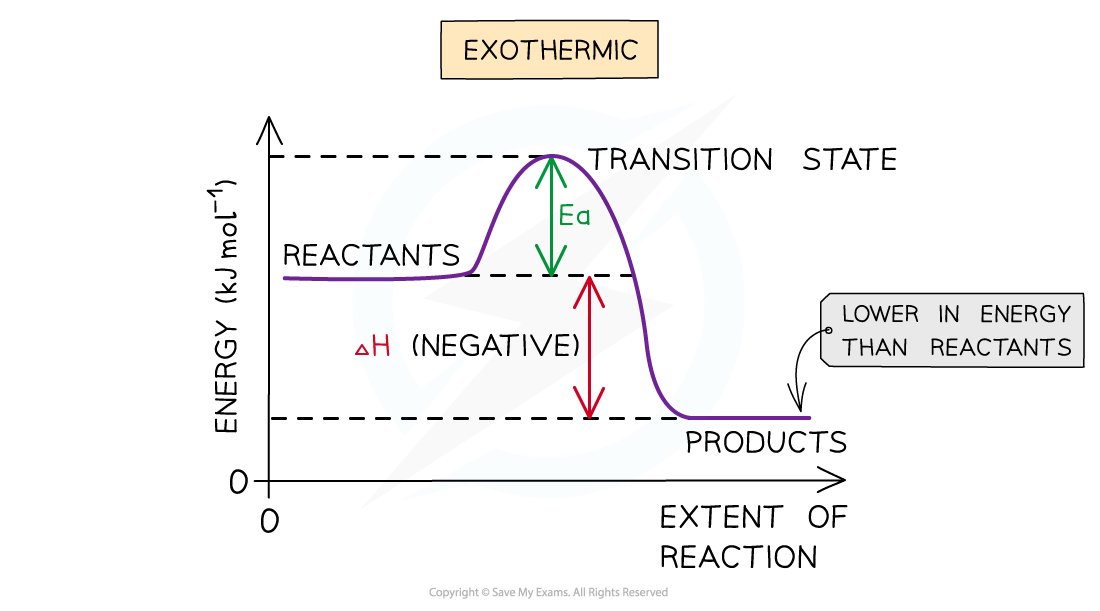
The energy level diagram for exothermic reactions. ∆H is negative because the system (reactants) loses energy to the surroundings.
Endothermic reaction
- In an endothermic reaction, the reactants are lower in energy than the products
- The reactants are therefore further away in energy to the transition state
- This means that endothermic reactions have a higher activation energy compared to exothermic reactions
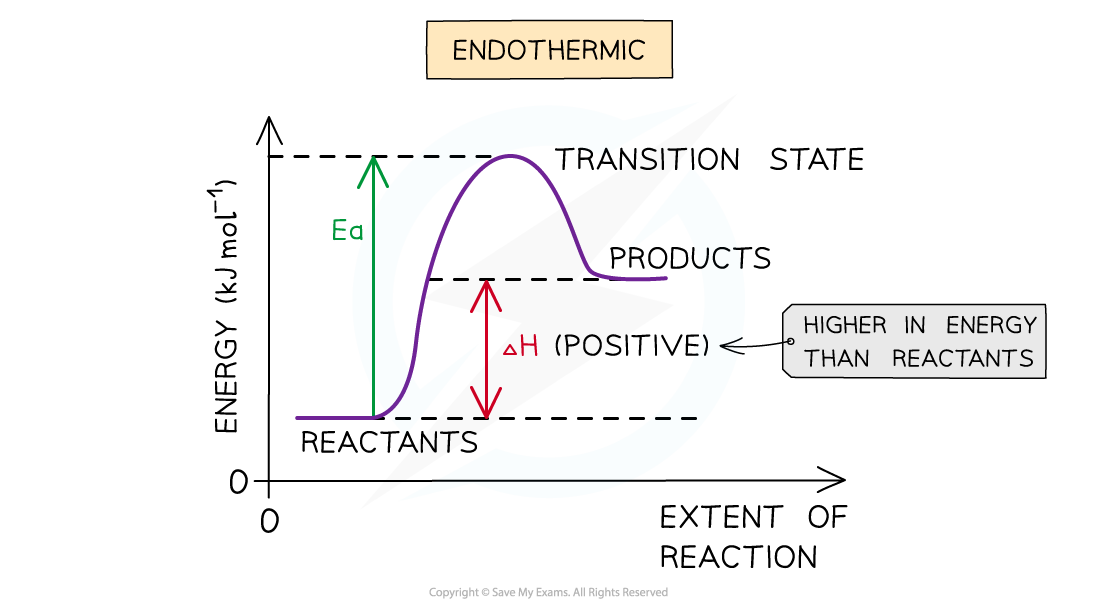
The energy level diagram for endothermic reactions. The system (reactant substances) have gained energy so ∆H is positive.
Worked Example
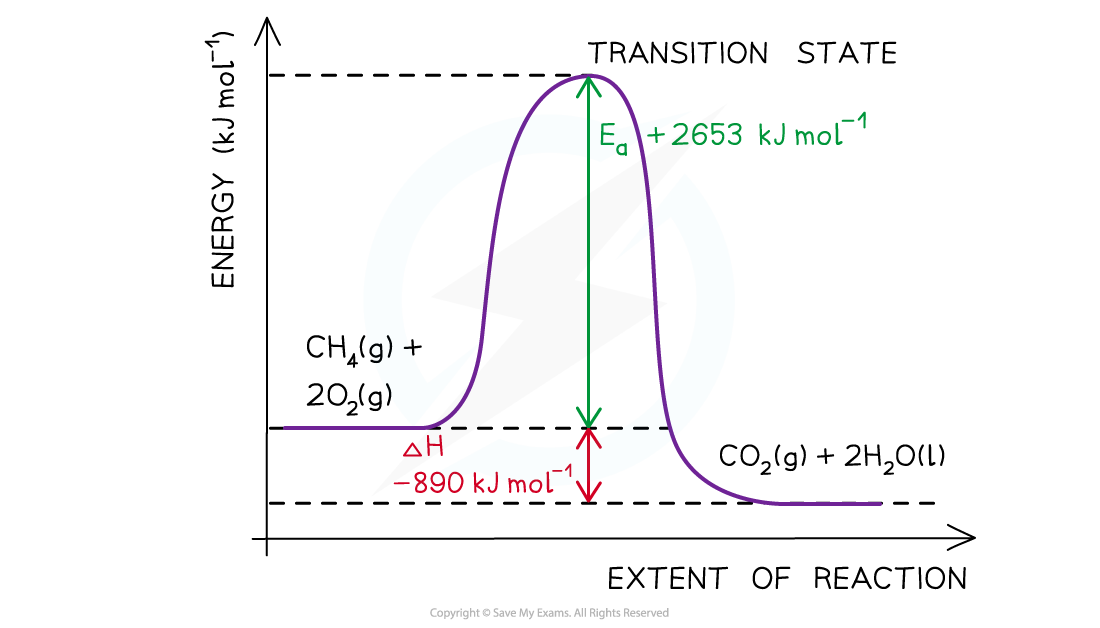
Drawing energy level diagrams of the combustion of methaneThe Ea and ΔH for the complete combustion of methane are +2653 kJ mol-1 and -890 kJ mol-1 respectively. Draw the energy level diagram for this reaction.
Answer
Step 1: The chemical equation for the complete combustion of methane is:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
Step 2: Combustion reactions are always exothermic (ΔH is negative) so the reactants should be drawn higher in energy than the products
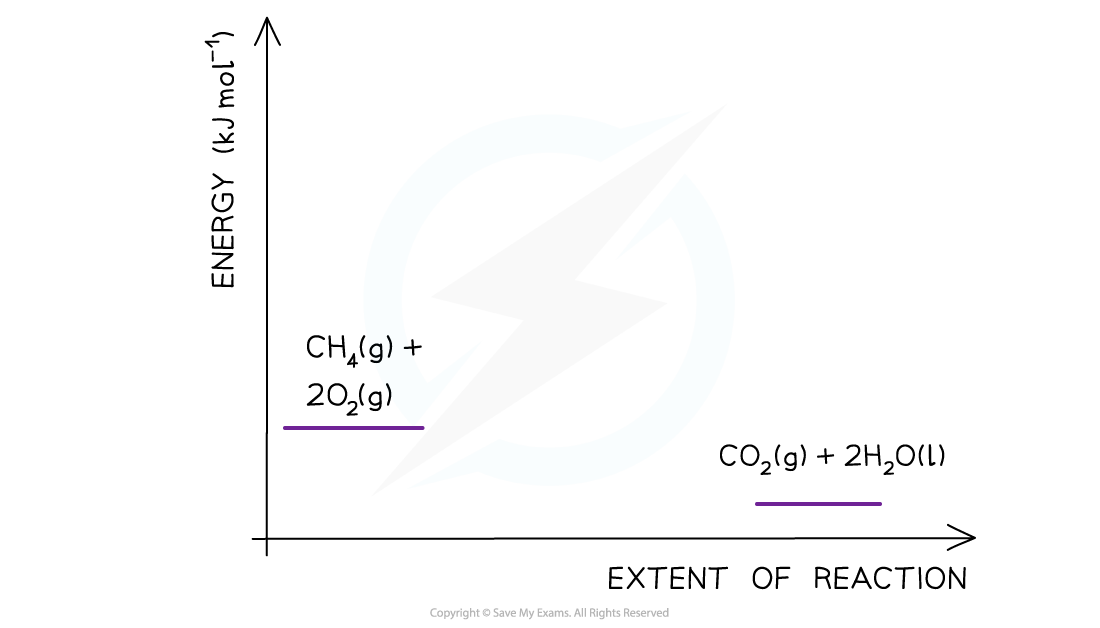
Step 3: Draw the curve in the energy level diagram clearly showing the transition state
Step 4: Draw arrows to show the Ea and ΔH including their values
Worked Example
Determining the activation energyΔH for a reaction is +70 kJ mol-1 and Ea for the reverse reaction is +20 kJmol-1. Use the reaction pathway diagram below to determine the Ea for the forward reaction.
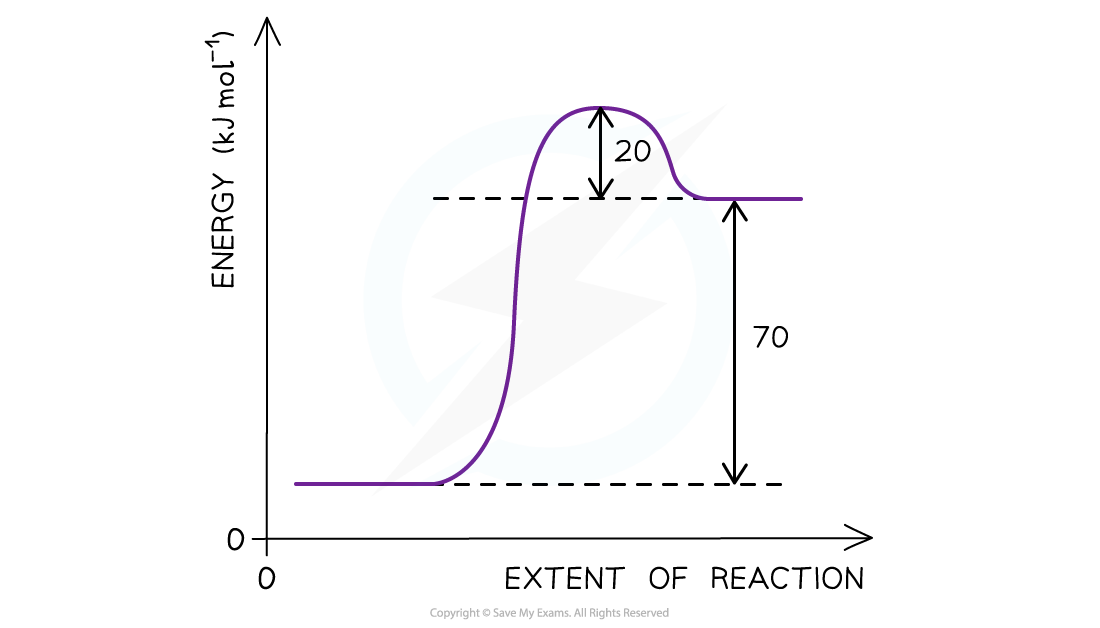
The reaction pathway diagram for a reversible reaction
Answer
-
- The Ea is the energy difference from the energy level of the reactants to the top of the ‘hump’
- Ea (forward reaction) = (+70 kJ mol-1) + (+ 20 kJ mol-1 ) = +90 kJ mol-1
Exam Tip
The activation energy is the energy difference from reactants to transition state.The enthalpy change of the reaction is the energy difference from reactants to products.Remember to label the axis of the energy level diagrams!
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





