- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.4.7 Properties of Ionic Compounds
Ionic Lattice Structures
- Most ionic, metallic and covalent solids are crystalline lattices
- The ions, atoms or molecules are arranged in a regular and repeating arrangement
Giant ionic lattices
- An ionic bond is an electrostatic force of attraction between a positively charged metal (cation) ion and a negatively charged non-metal (anion) ion
- The metal becomes positively charged as it transfers electrons to the non-metal which then becomes negatively charged
- When an ionic compound is formed, the attraction between the ions happens in all directions
- Ionic compounds are arranged in giant ionic lattices (also called giant ionic structures)
- The type of lattice formed depends on the sizes of the positive and negative ions which are arranged in an alternating fashion
- The ionic lattice of MgO and NaCl are cubic
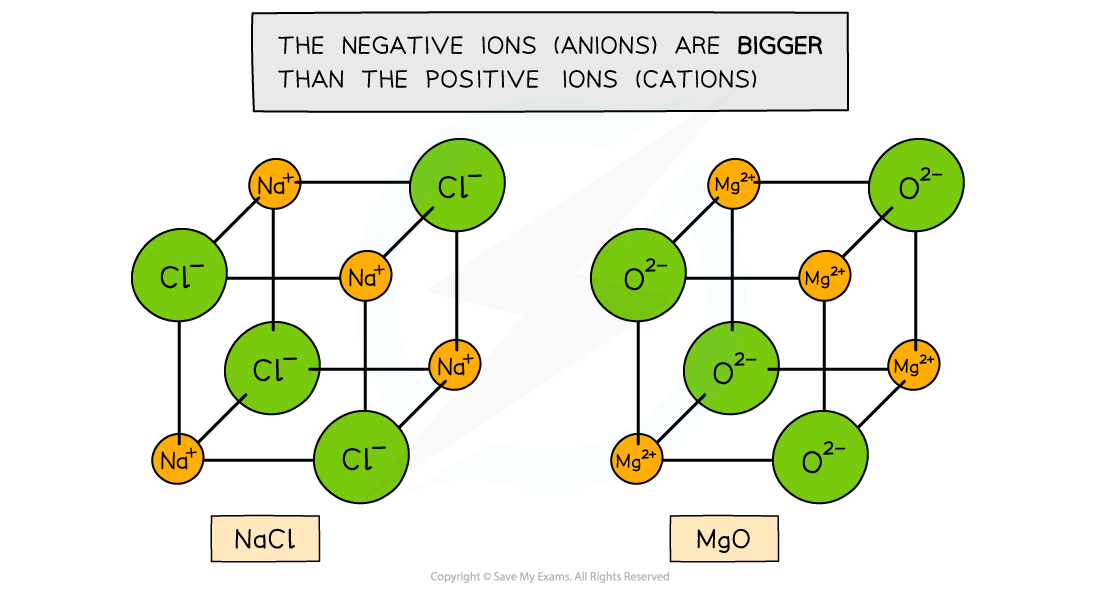
Ionic lattices of the ionic compounds NaCl and MgO
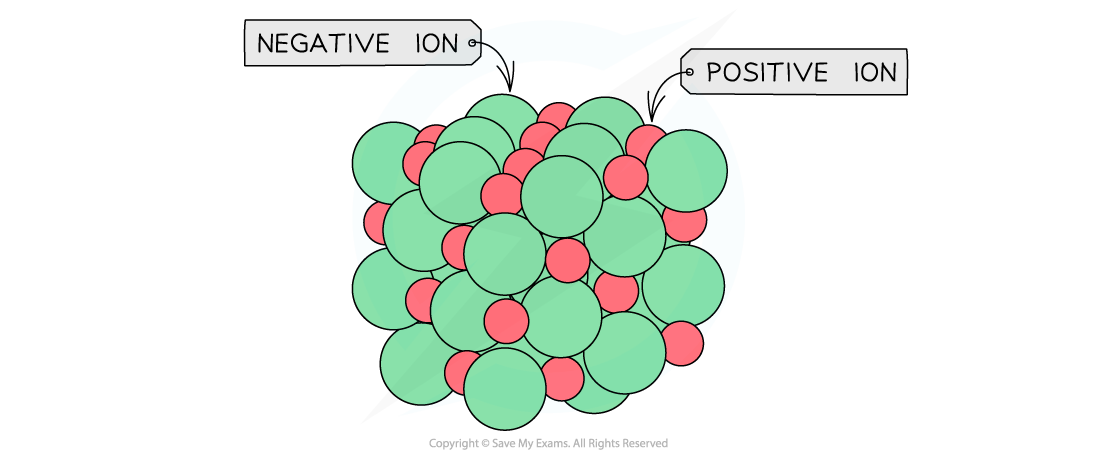
General ionic lattice which shows the actual packing of the ions
Exam Tip
It is important that you can state and fully explain the different properties which arise based on the structure and bonding present in a substance
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





