- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.3.4 Protein Interactions
Proteins: Interactions & Shape
- A polypeptide chain will fold differently due to the interactions (and hence the bonds that form) between R groups. The three-dimensional configuration that forms is called the tertiary structure of a protein
- Each of the twenty amino acids that make up proteins has a unique R group and therefore many different interactions can occur creating a vast range of protein configurations and therefore functions
- Within tertiary structured proteins are the following bonds:
- Strong covalent disulphide
- Weak hydrophobic interactions
- Weak hydrogen
- Ionic
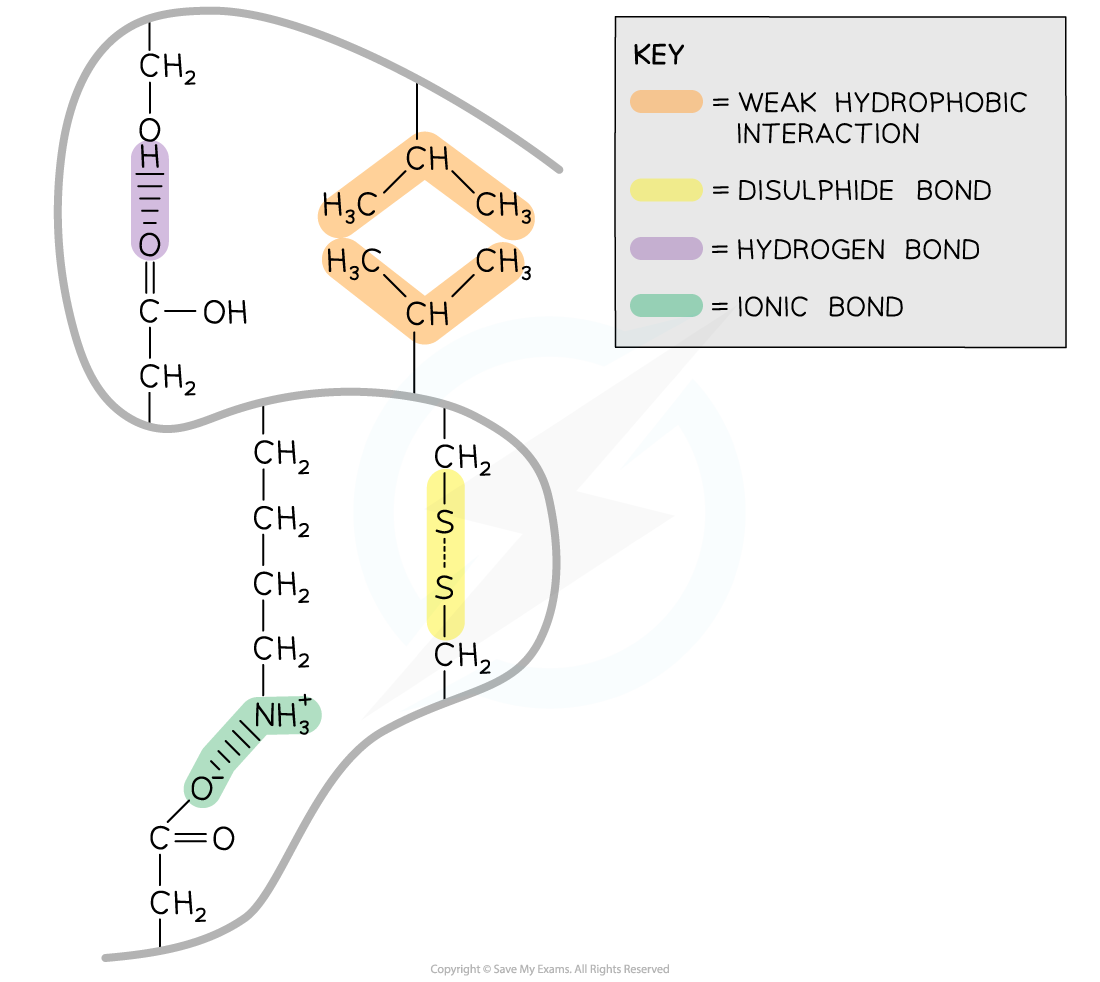
The interactions that occur between the R groups of amino acids determines the shape and function of a protein. These interactions are found within tertiary structures of proteins
Disulphide
- Disulphide bonds are strong covalent bonds that form between two cysteine R groups (as this is the only amino acid with an available sulphur atom in its R group)
- These bonds are the strongest within a protein, but occur less frequently, and help stabilise the proteins
- These are also known as disulphide bridges
- Can be broken by oxidation
- Disulphide bonds are common in proteins secreted from cells eg. insulin
Ionic
- Ionic bonds form between positively charged (amine group -NH3+) and negatively charged (carboxylic acid -COO-) R groups
- Ionic bonds are stronger than hydrogen bonds but they are not common
- These bonds are broken by pH changes
Hydrogen
- Hydrogen bonds form between strongly polar R groups. These are the weakest bonds that form but the most common as they form between a wide variety of R groups
Hydrophobic interactions
- Hydrophobic interactions form between the non-polar (hydrophobic) R groups within the interior of proteins
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





