- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.3.3 Protein Structure & Function
Proteins: Structures & Functions
- There are four levels of structure in proteins, three are related to a single polypeptide chain and the fourth level relates to a protein that has two or more polypeptide chains
- Polypeptide or protein molecules can have anywhere from 3 amino acids (Glutathione) to more than 34,000 amino acids (Titan) bonded together in chains
Primary
- The sequence of amino acids bonded by covalent peptide bonds is the primary structure of a protein
- DNA of a cell determines the primary structure of a protein by instructing the cell to add certain amino acids in specific quantities in a certain sequence. This affects the shape and therefore the function of the protein
- The primary structure is specific for each protein (one alteration in the sequence of amino acids can affect the function of the protein)
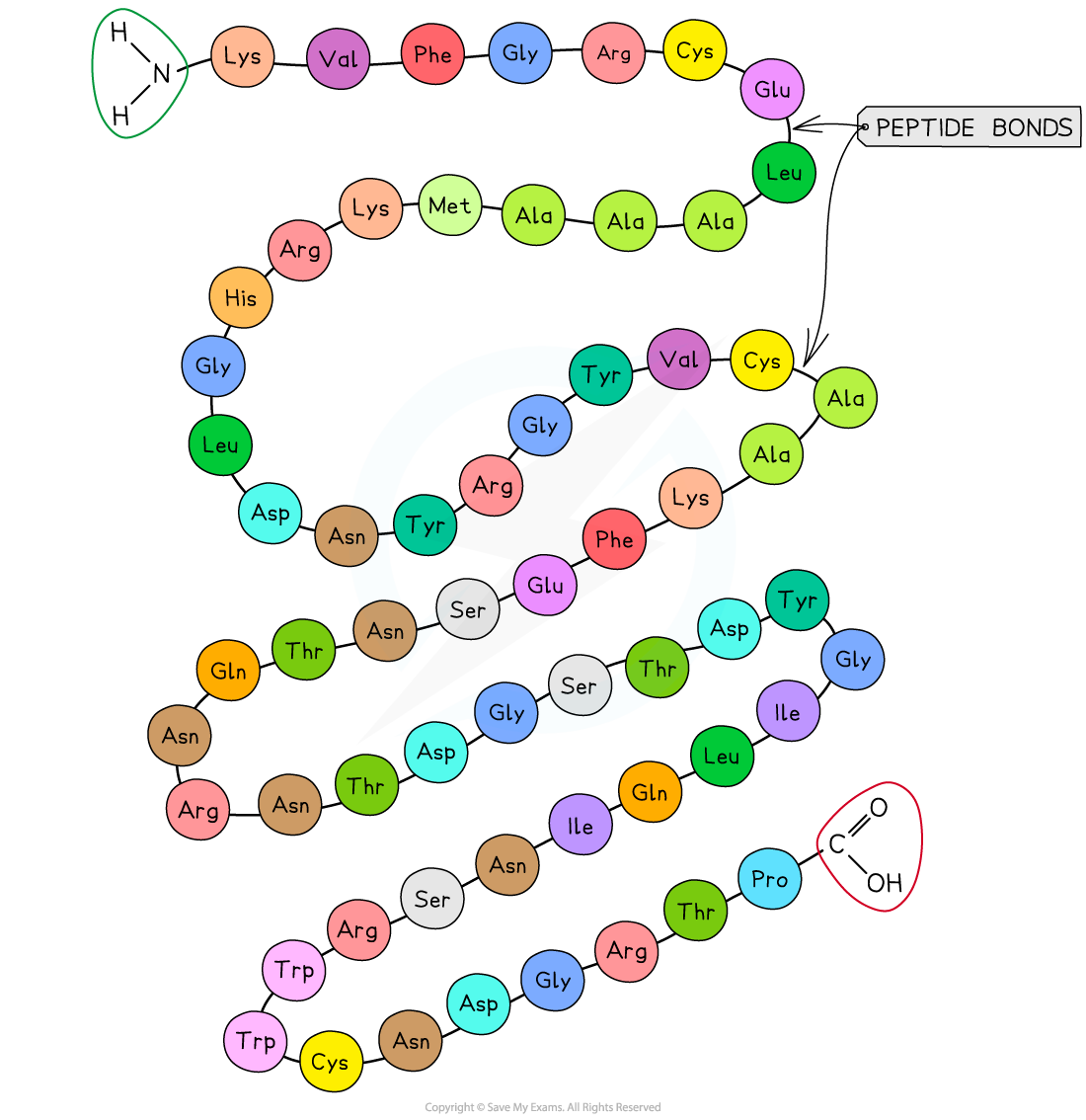
The primary structure of a protein. The three-letter abbreviations indicate the specific amino acid (there are 20 commonly found in cells of living organisms)
Secondary
- The secondary structure of a protein occurs when the weak negatively charged nitrogen and oxygen atoms interact with the weak positively charged hydrogen atoms to form hydrogen bonds
- There are two shapes that can form within proteins due to the hydrogen bonds:
- α-helix
- β-pleated sheet
- The α-helix shape occurs when the hydrogen bonds form between every fourth peptide bond (between the oxygen of the carboxyl group and the hydrogen of the amine group)
- The β-pleated sheet shape forms when the protein folds so that two parts of the polypeptide chain are parallel to each other enabling hydrogen bonds to form between parallel peptide bonds
- Most fibrous proteins have secondary structures (e.g. collagen and keratin)
- The secondary structure only relates to hydrogen bonds forming between the amino group and the carboxyl group (the ‘protein backbone’)
- The hydrogen bonds can be broken by high temperatures and pH changes
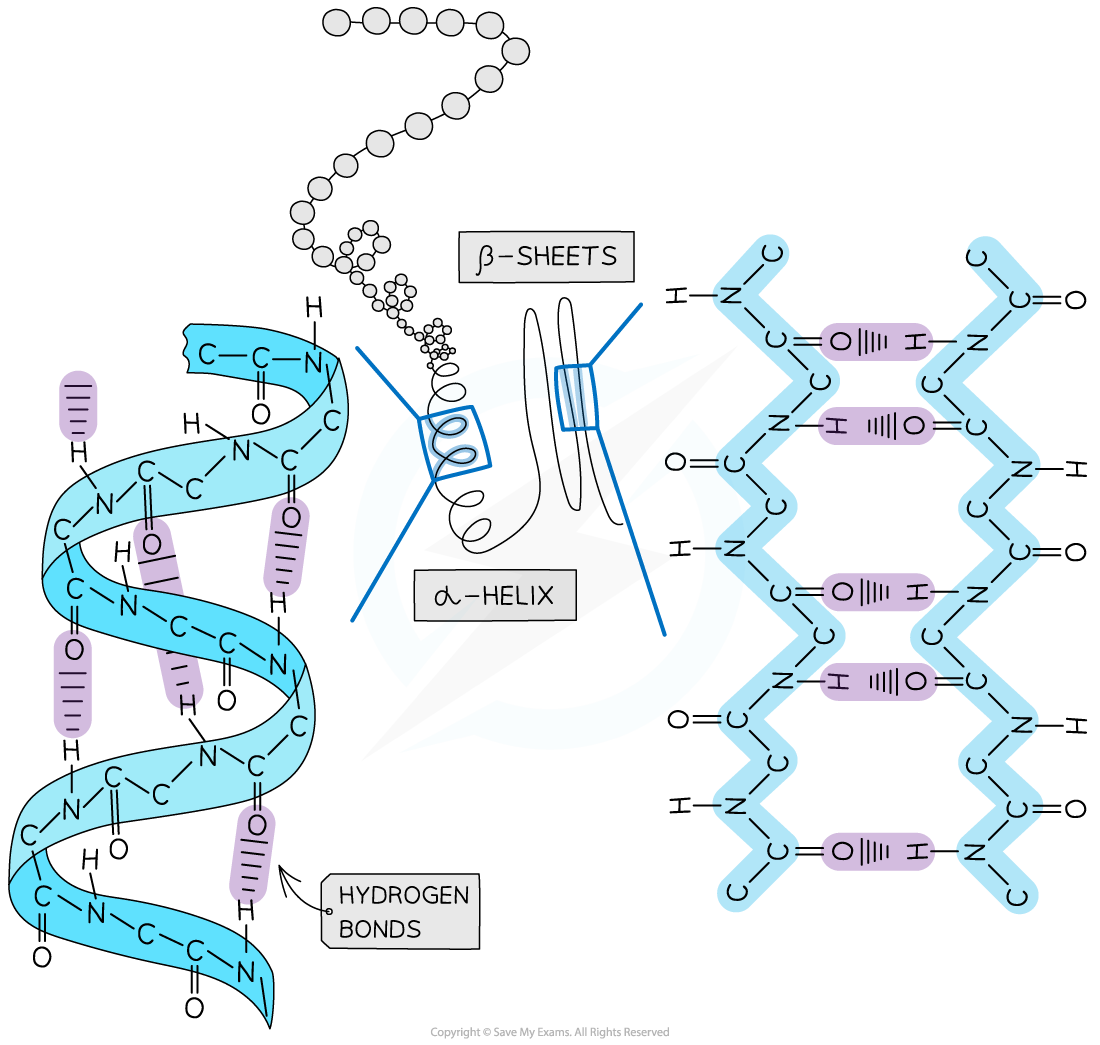
The secondary structure of a protein with the α-helix and β-pleated sheet shapes highlighted. The magnified regions illustrate how the hydrogen bonds form between the peptide bonds
Tertiary
- Further conformational change of the secondary structure leads to additional bonds forming between the R groups (side chains)
- The additional bonds are:
- Hydrogen (these are between R groups)
- Disulphide (only occurs between cysteine amino acids)
- Ionic (occurs between charged R groups)
- Weak hydrophobic interactions (between non-polar R groups)
- This structure is common in globular proteins
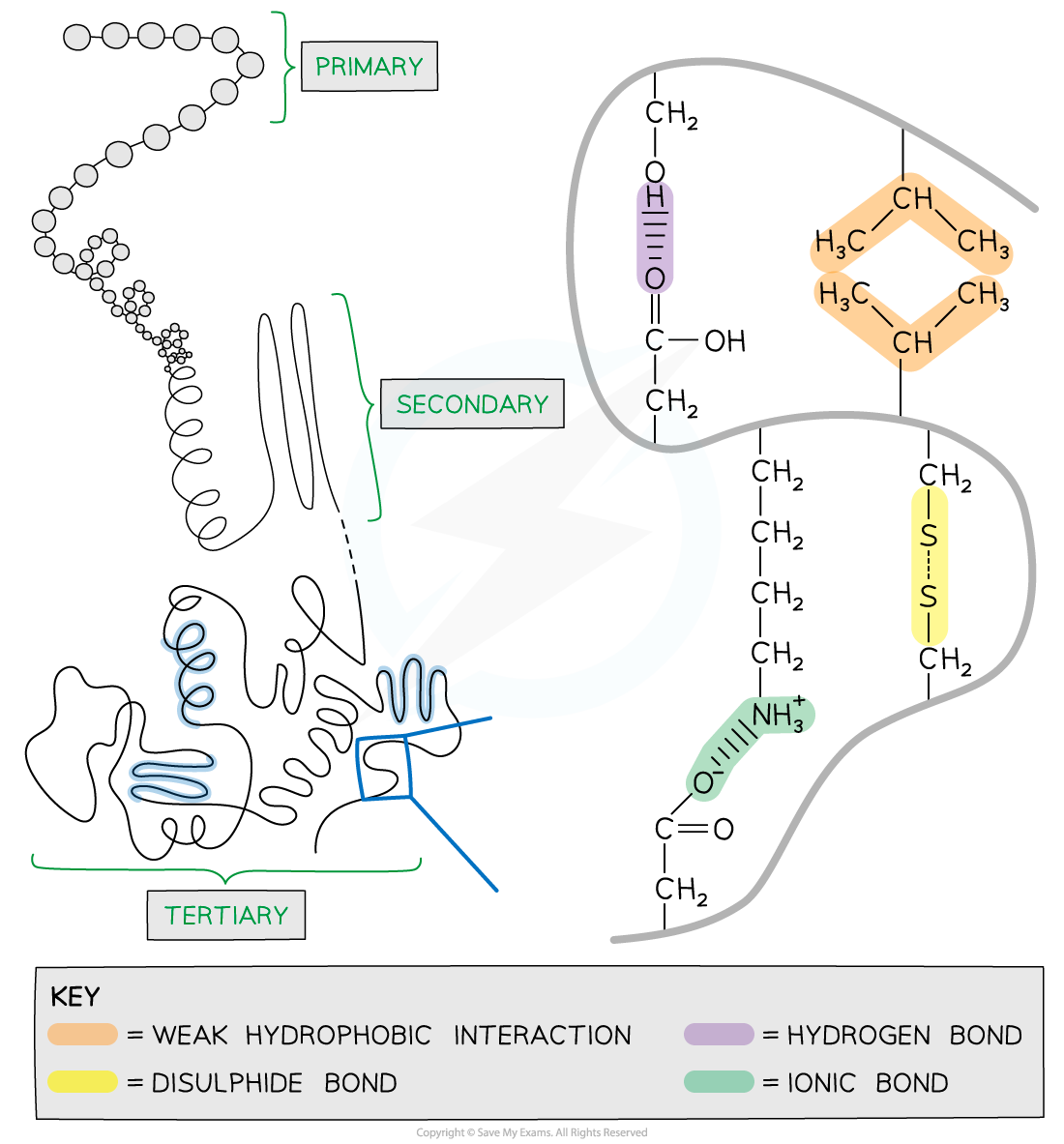
The tertiary structure of a protein with hydrogen bonds, ionic bonds, disulphide bonds and hydrophobic interactions formed between the R groups of the amino acids
Quaternary
- Occurs in proteins that have more than one polypeptide chain working together as a functional macromolecule, for example, haemoglobin
- Each polypeptide chain in the quaternary structure is referred to as a subunit of the protein
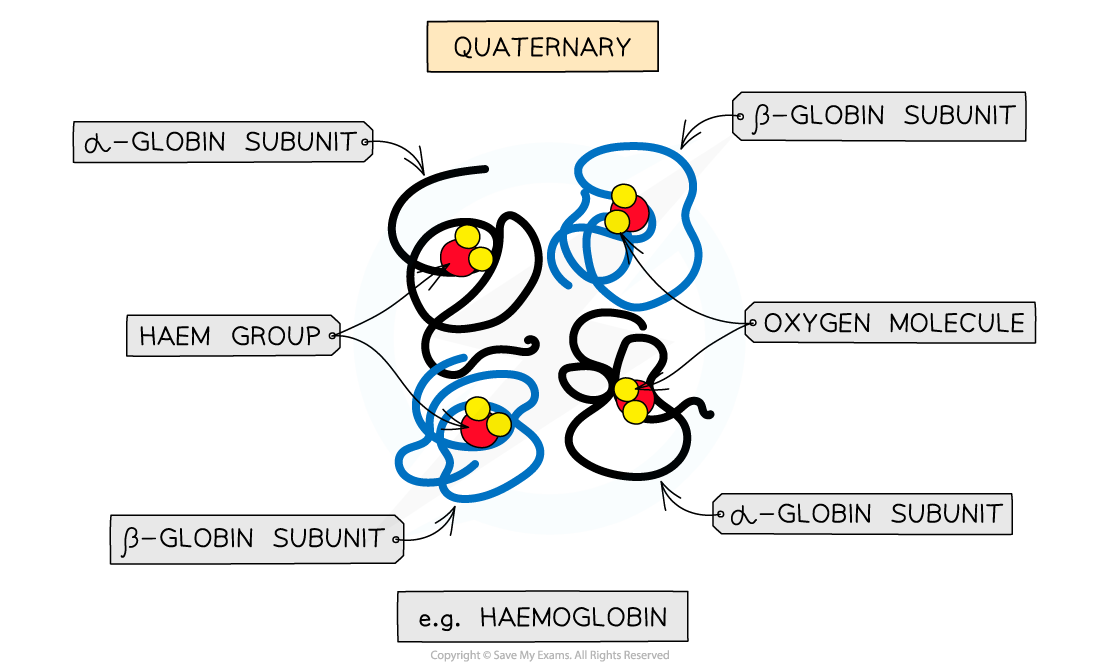
The quaternary structure of a protein. This is an example of haemoglobin which contains four subunits (polypeptide chains) working together to carry oxygen
Summary of Bonds in Proteins Table
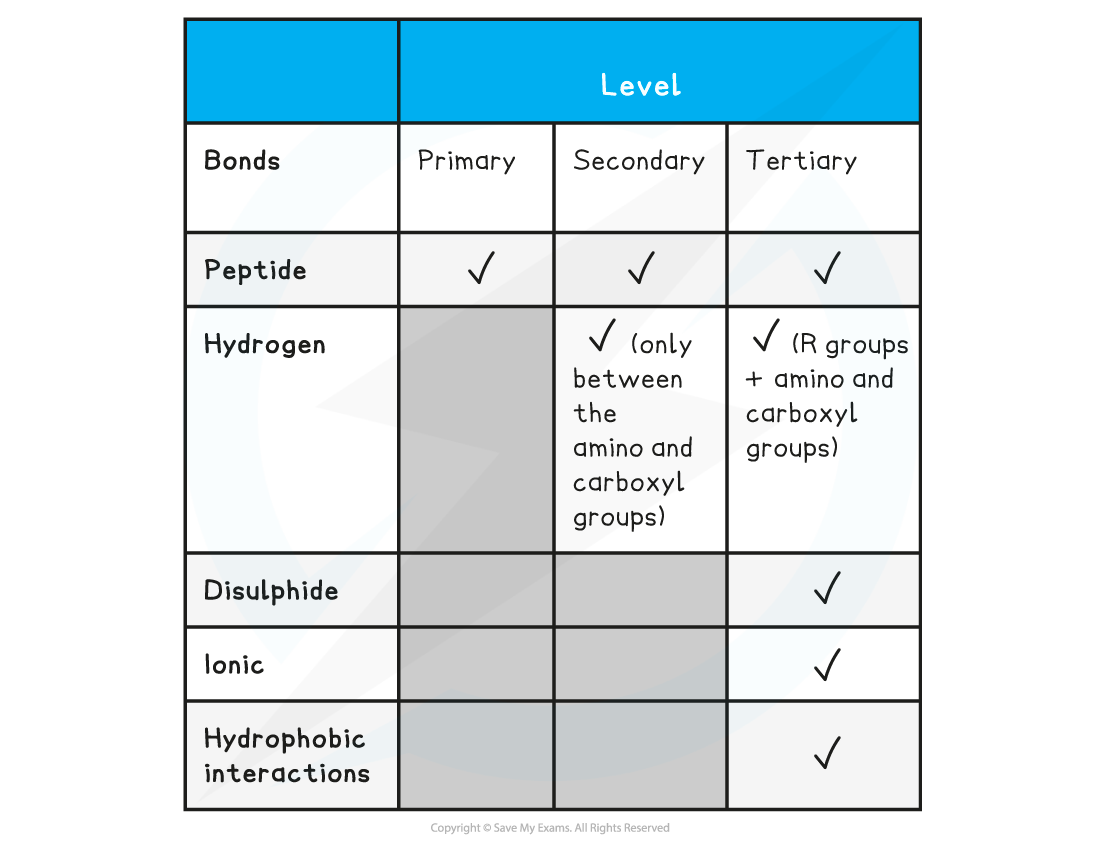
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





