- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Biology复习笔记1.1.2 Biological Molecules: Reactions
Biological Molecules: Reactions
- A covalent bond is the sharing of two or more electrons between two atoms
- The electrons can be shared equally forming a nonpolar covalent bond or unequally (where an atom can be more electronegative δ-) to form a polar covalent bond
- Generally each atom will form a certain number of covalent bonds due to the number of free electrons in the outer orbital e.g. H = 1 bond, C = 4 bonds
- Covalent bonds are very stable as high energies are required to break the bonds
- Multiple pairs of electrons can be shared forming double bonds (e.g. unsaturated fats C=C) or triple bonds
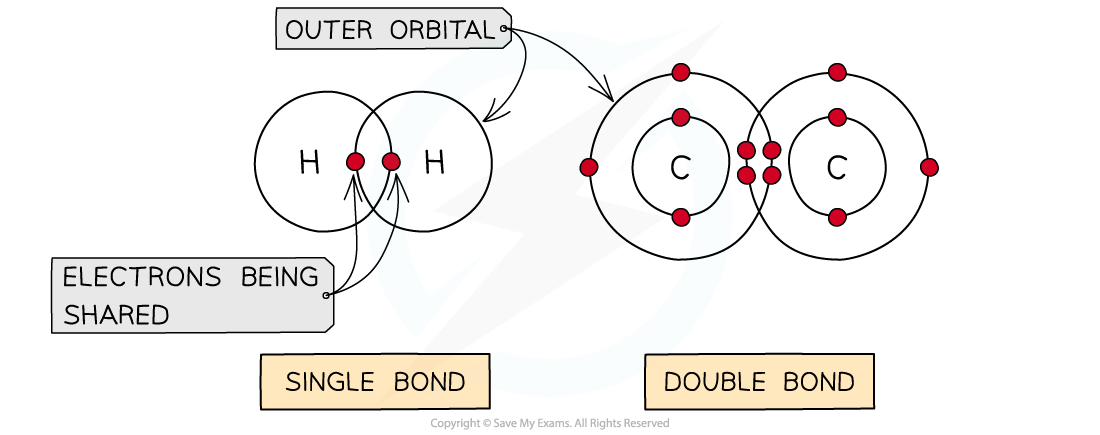
Different types of covalent bonds
- When two monomers are close enough that their outer orbitals overlap this results in their electrons being shared and a covalent bond forming. If more monomers are added then polymerisation occurs (and / or a macromolecule forms)
Condensation
- Also known as dehydration synthesis (‘to put together while losing water’)
- A condensation reaction occurs when monomers combine together by covalent bonds to form polymers (polymerisation) or macromolecules (lipids) and water is removed
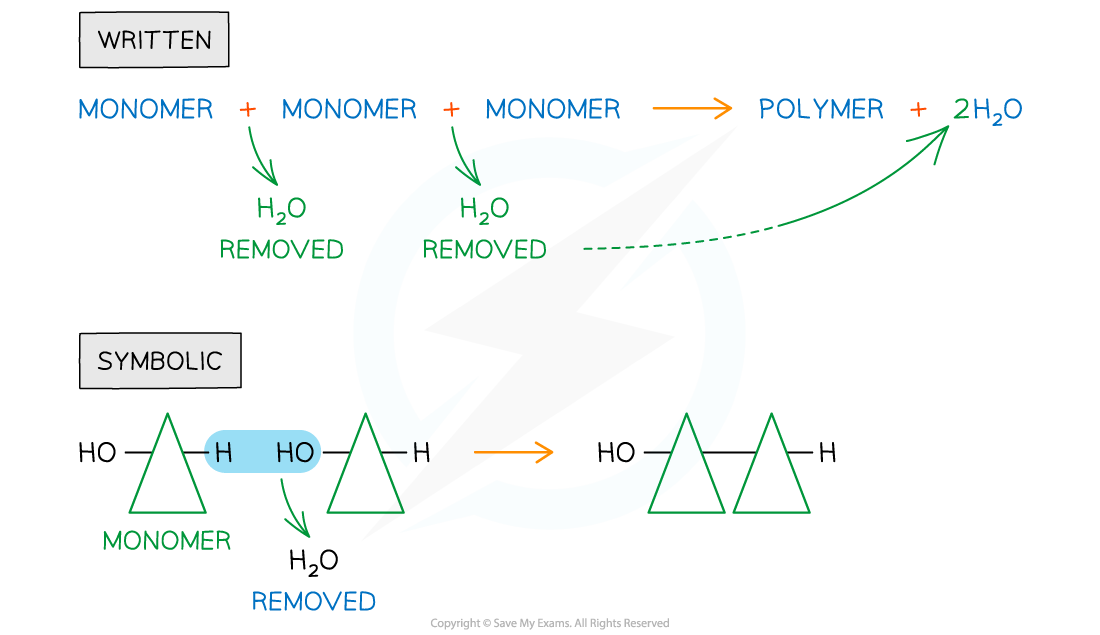
Written and symbolic illustrations of the removal of water to form a covalent bond between two or more monomers during a condensation reaction
Hydrolysis
- Hydrolysis means ‘lyse’ (to break) and ‘hydro’ (with water)
- In the hydrolysis of polymers, covalent bonds are broken when water is added
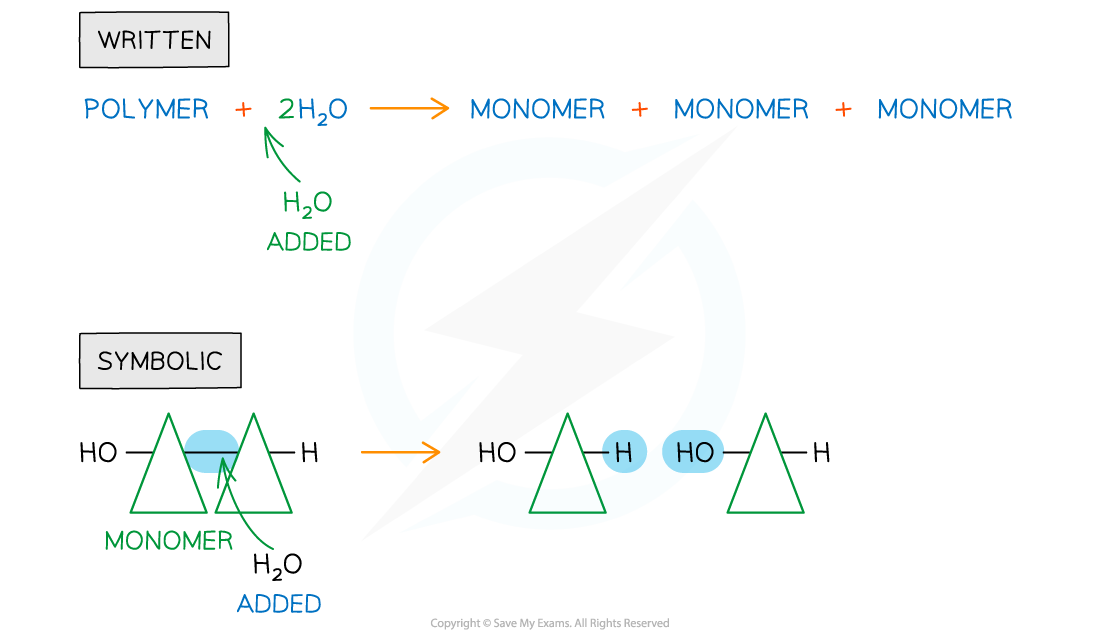
Written and symbolic illustrations of the addition of water to break down covalent bond/s during a hydrolysis reaction
Covalent Bonds in Organic Molecules Table
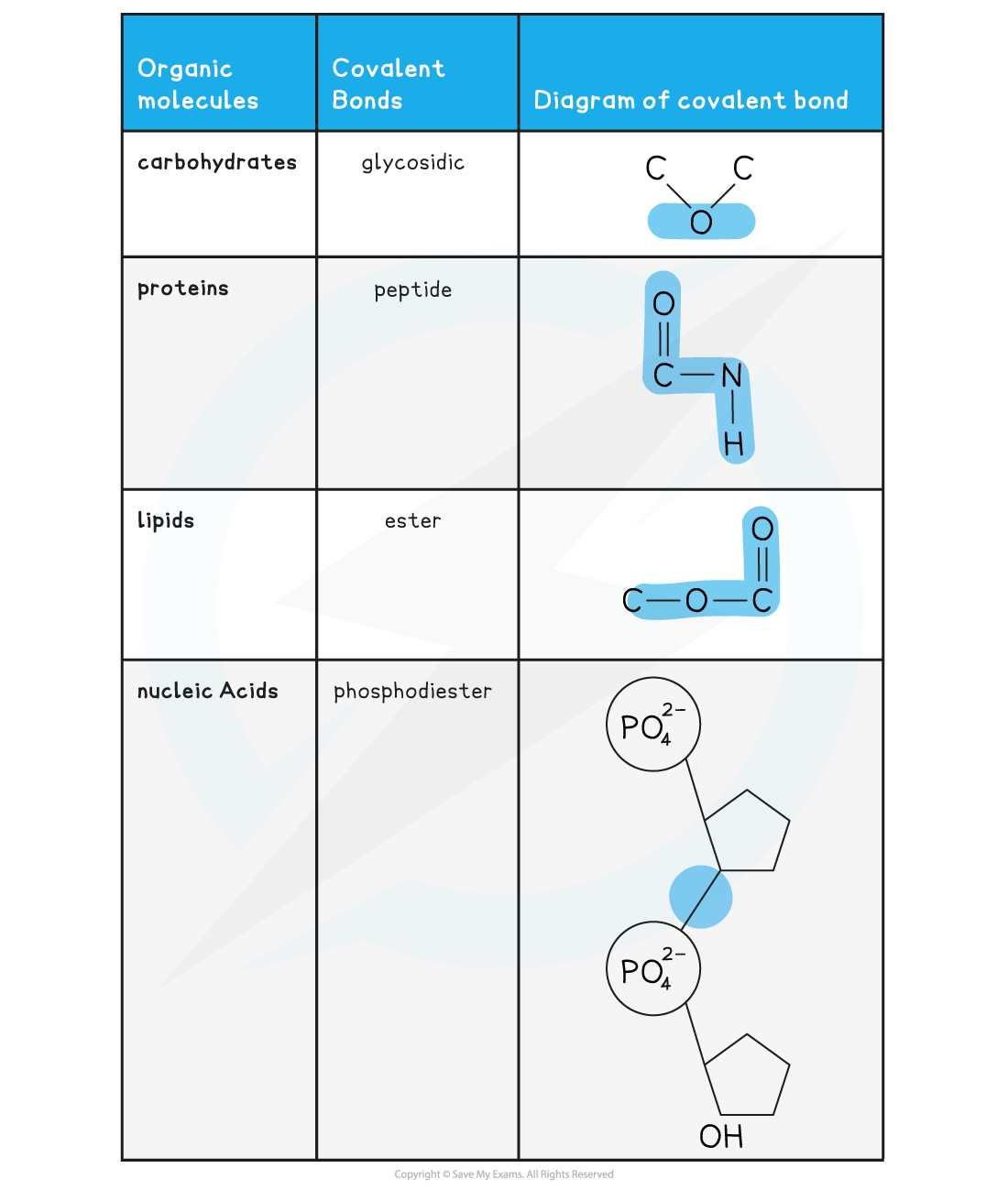
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





