- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.3.1 Carboxylic Acids
Carboxylic Acids
Carboxylic acids
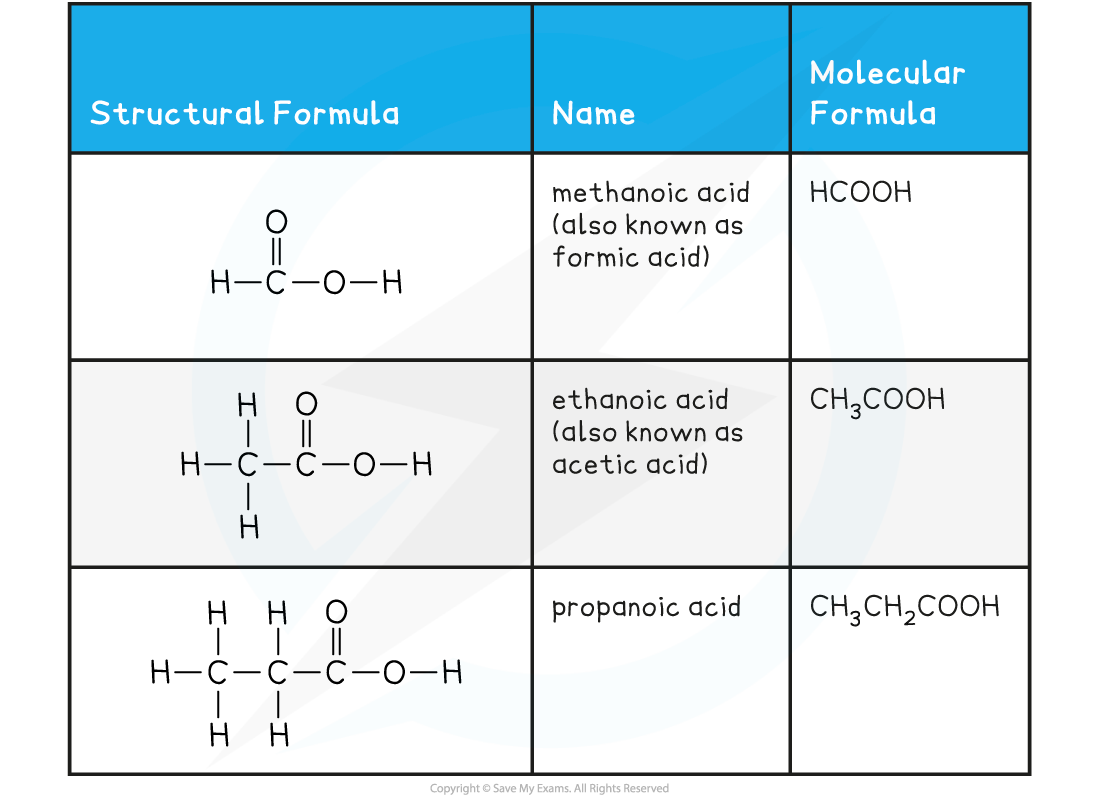
- Carboxylic acid is the name given to the family of compounds that contain the carboxyl functional group, -COOH
- The general formula of a carboxylic acid is CnH2n+1COOH which can be shortened to just RCOOH
- (In some countries, this family is also called alkanoic acid)
- The nomenclature of carboxylic acid follows the pattern alkan + oic acid, e.g. propanoic acid
- There is no need to use numbers in the name as the carboxyl group, COOH, is always on the number 1 carbon atom
Carboxylic Acids Examples Table
Weak Acids
- Carboxylic acids with fewer than six carbon atoms per molecule are water-soluble
- This is because water molecules can hydrogen-bond with the functional group
- In aqueous solution they are only slightly ionised, to give low concentrations of hydronium ions and alkanoate ions (often called carboxylate ions)

Carboxylic acids are weak acids that do not fully dissociate in water, the position of the equilibrium lies to the left
- This partial ionisation in solution means that carboxylic acids are weak acids
- This means that the position of the equilibrium lies to the left and that the concentration of H+ is much smaller than the concentration of the carboxylic acid
- However, the concentration of hydrogen ions is sufficient to react with an aqueous solution of sodium carbonate or sodium hydrogen carbonate to produce carbon dioxide
- These reactions are a useful test for the possible presence of a carboxylic acid:
- Sodium carbonate: 2RCOOH + Na2CO3 → 2RCOO-Na+ + CO2 + H2O
- Ionic equation with carbonates: 2RCOOH + CO32- → 2RCOO- + CO2 + H2O
- Sodium hydrogen carbonate: RCOOH + NaHCO3 → RCOO-Na+ + CO2 + H2O
- Ionic equation with hydrogen carbonates: RCOOH + HCO3- → RCOO- + CO2 + H2O
Preparation of esters
- Esters are formed from the condensation reaction between an alcohol and carboxylic acid in the presence of a concentrated strong acid catalyst e.g. sulfuric or hydrochloric acid
- This reaction is often referred to as esterification
- A condensation reaction involves the elimination of a small molecule not always water
- Esterification is one example of a condensation reaction as water is eliminated from the acid and alcohol reacting together

转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





