- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.10.1 Principles of NMR
Principles of NMR
- Nuclear Magnetic Resonance (NMR) spectroscopy is used for analysing organic compounds
- NMR analysis can provide information about the positions of 13C and 1H atoms in a molecule
- All samples are measured against a reference compound – Tetramethylsilane (TMS)
- TMS shows a single sharp peak on NMR spectra, at a value of zero
- Sample peaks are then plotted as a ‘shift’ away from this reference peak
- This gives rise to ‘chemical shift’ values for protons on the sample compound
- Chemical shifts are measured in parts per million (ppm)
Exam Tip
You are often asked in exam questions why tetramethylsilane (TMS) is a suitable solvent for NMR analysis. Try to remember that TMS is:
- Non toxic.
- Does not react with the sample.
- Easily separated from the sample molecule due to its low boiling point.
- Produces one strong, sharp absorption peak on the spectrum.
Worked Example
Draw the structural formula of TMS
Answer:
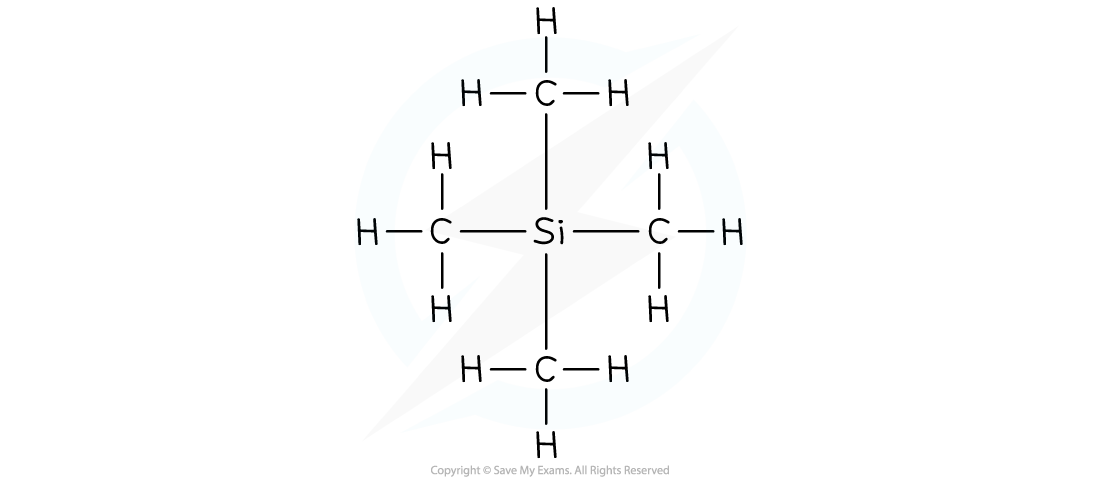
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





