- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记3.3.1 Reactivity of Halogenoalkanes
Reactivity of Halogenoalkanes
- The halogenoalkanes have different rates of substitution reactions
- Since substitution reactions involve breaking the carbon-halogen bond the bond energies can be used to explain their different reactivities
Halogenoalkane Bond Energy Table
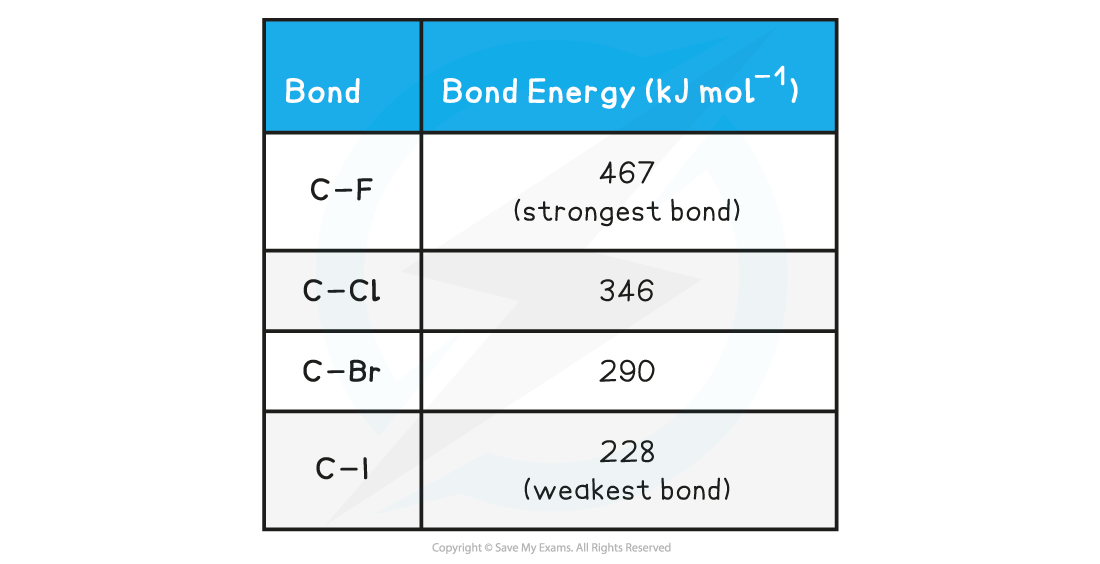
- The table above shows that the C-I bond requires the least energy to break, and is therefore the weakest carbon-halogen bond
- During substitution reactions the C-I bond will therefore heterolytically break as follows:
R3C-I + OH- → R3C-OH + I-
halogenoalkane alcohol
- The C-F bond, on the other hand, requires the most energy to break and is, therefore, the strongest carbon-halogen bond
- Fluoroalkanes will therefore be less likely to undergo substitution reactions
Aqueous silver nitrate
- Reacting halogenoalkanes with aqueous silver nitrate solution will result in the formation of a precipitate
- The rate of formation of these precipitates can also be used to determine the reactivity of the halogenoalkanes
Halogenoalkane Precipitates Table
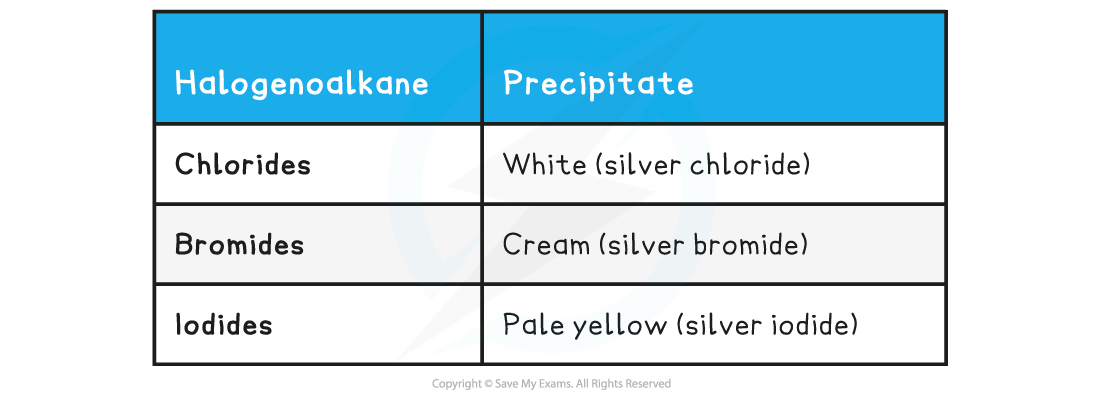
- The formation of the pale yellow silver iodide is the fastest (fastest nucleophilic substitution reaction) whereas the formation of the silver fluoride is the slowest (slowest nucleophilic substitution reaction)
- This confirms that fluoroalkanes are the least reactive and iodoalkanes are the most reactive halogenoalkanes
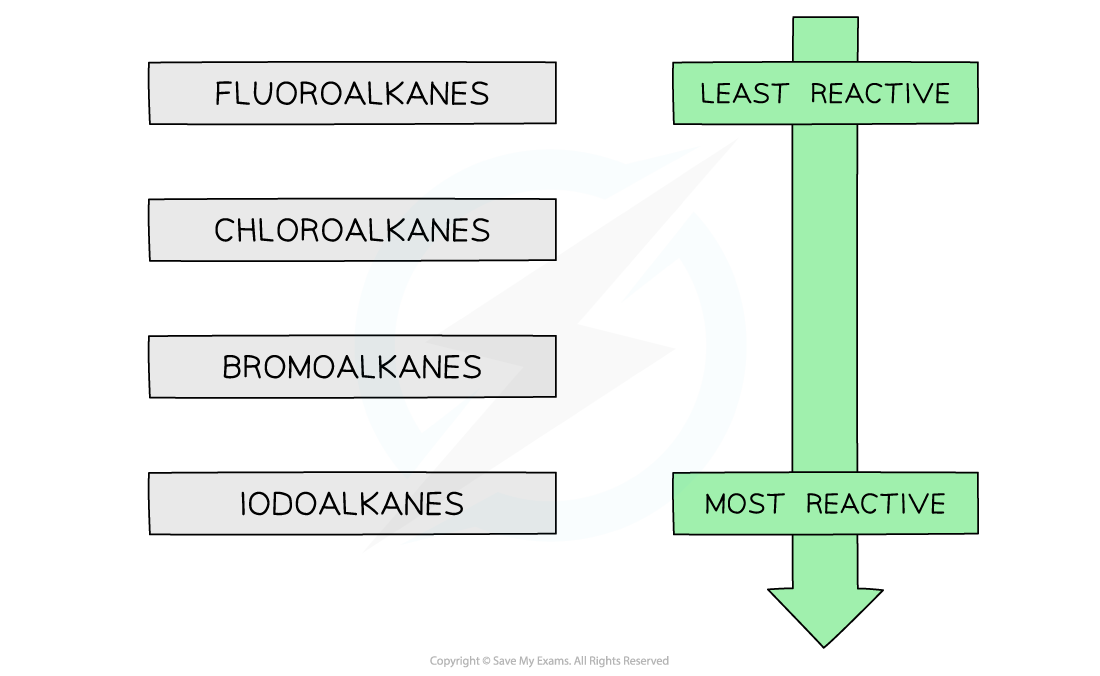
The trend in reactivity of halogenoalkanes
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





