- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.9.1 Oxidation & Reduction
Oxidation & Reduction
- There are three definitions of oxidation and reduction used in different branches of chemistry
- Oxidation and reduction can be used to describe any of the following processes
Definitions and Examples of Oxidation & Reduction
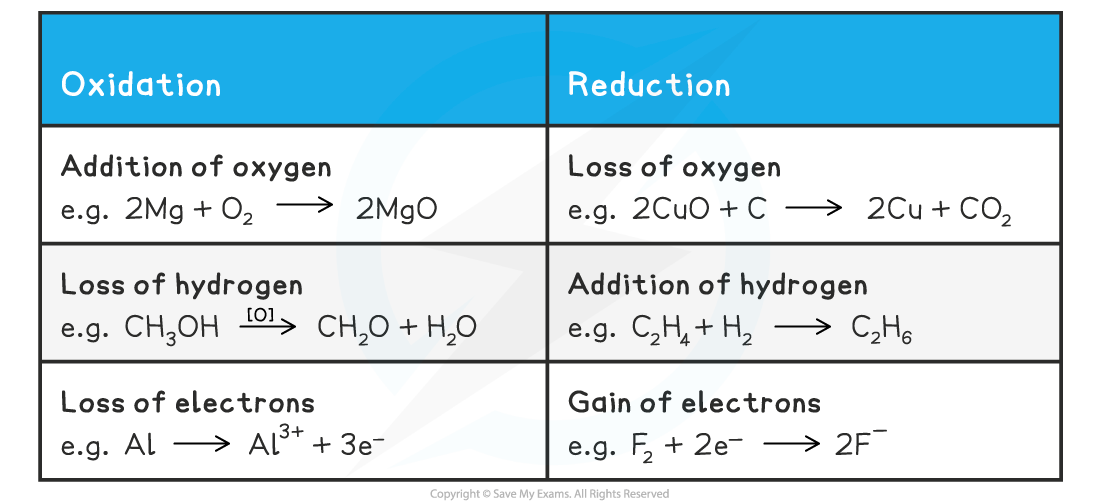
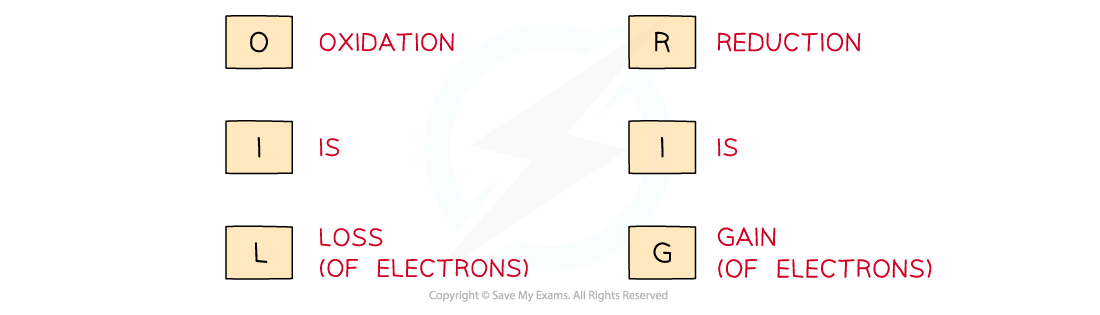
Use the acronym "Oil Rig" to help you remember the definitions of oxidation and reduction
Oxidation States
- The oxidation state of an atom is the charge that would exist on an individual atom if the bonding were completely ionic
- It is like the electronic ‘status’ of an element
- Oxidation states are used to
- Tell if oxidation or reduction has taken place
- Work out what has been oxidised and/or reduced
- Construct half equations and balance redox equations
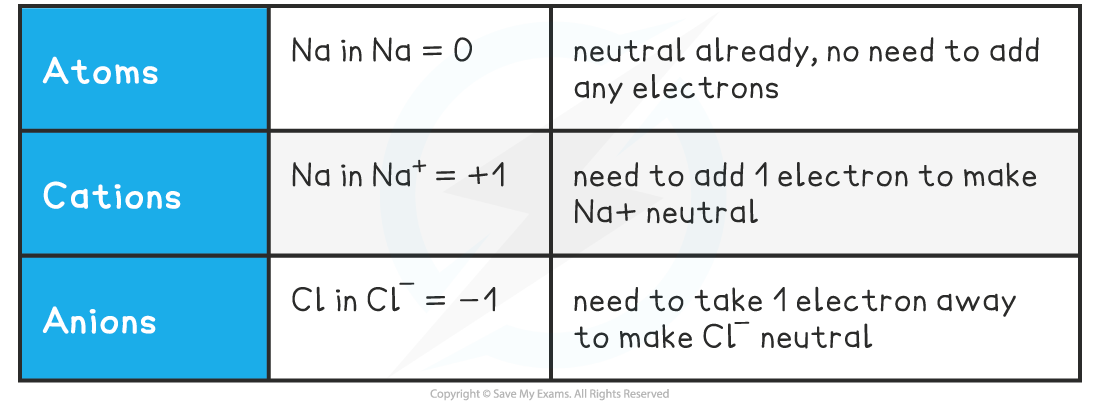
Oxidation States of Simple Ions
Worked Example
What are the oxidation states of the elements in the following species?a) C b) Fe3+ c) Fe2+d) O2- e) He f) Al3+
Answers:
a) 0 b) +3 c) +2 d) -2 e) 0 f) +3
- So, in simple ions, the oxidation stateof the atom is the charge on the ion:
- Na+, K+, H+ all have an oxidation state of +1
- Mg2+, Ca2+, Pb2+ all have an oxidation state of +2
- Cl–, Br–, I– all have an oxidation state of -1
- O2-, S2- all have an oxidation state of -2
Exam Tip
Oxidation state and oxidation number are often used interchangeably, though IUPAC does not distinguish between the two terms.Oxidation states are represented by Roman numerals according to IUPAC
Oxidising & Reducing Agents
Oxidising agent
- An oxidising agent is a substance that oxidises another atom or ion by causing it to lose electrons
- An oxidising agent itself gets reduced – it gains electrons
- Therefore, the oxidation state of the oxidising agent decreases

Example of an oxidising agent in a chemical reaction
Reducing agent
- A reducing agent is a substance that reduces another atom or ion by causing it to gain electrons
- A reducing agent itself gets oxidised – it loses/donates electrons
- Therefore, the oxidation state of the reducing agent increases

Example of a reducing agent in a chemical reaction
- For a reaction to be a redox reaction, there must be both an oxidising and reducing agent present
- Some substances can act both as oxidising and reducing agents - look at the two roles of H2O2 in the previous examples
- The role they take is dependent on what they are reacting with and the reaction conditions
Worked Example
Oxidising & reducing agentsFour reactions are shown. In which reaction is the species in bold acting as an oxidising agent?
- Cr2O72-+ 8H+ + 3SO32- → 2Cr3+ + 4H2O + 3SO42-
- Mg + Fe2+ → Mg2+ + Fe
- Cl2 + 2Br- → 2Cl- + Br2
- Fe2O3+ 3CO → 2Fe + 3CO2
Answer
The correct option is 2
-
- Oxidising agents are substances that oxidise other species, gain electrons and are themselves reduced.
- Write down the oxidation numbers of each species in the reaction
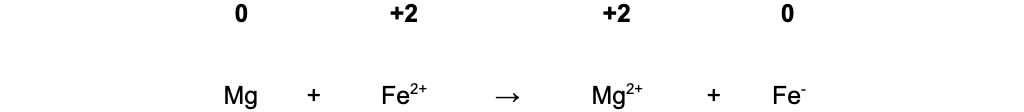
-
- In equation 2, Fe2+ oxidises Mg(0) to Mg2+(+2) and is itself reduced from Fe2+(+2) to Fe(0)
Roman numerals
- Roman numerals are used to show the oxidation states of transition metals which can have more than one oxidation state
- Iron can be both +2 and +3 so Roman numerals are used to distinguish between them
- Fe2+ in FeO is written as iron(II) oxide
- Fe3+ in Fe2O3 is written as iron(III) oxide
Worked Example
Systematic names of compoundsGive the full systematic names of the following compounds:
- FeCl2
- HClO4
- NO2
- Mg(NO3)2
- K2SO4
Answer
Answer 1: Iron(II) chloride: the oxidation state of 2 Cl atoms is -2 and FeCl2 has no overall charge so the oxidation state of Fe is +2
Answer 2: Chloric(VII) acid: the oxidation state of H is +1, 4 O atoms is -8 and HClO4 has no overall charge so the oxidation state of Cl is +7
Answer 3: Nitrogen(IV) oxide: the oxidation state of 2 O atoms is -4 and NO2 has no overall charge so the oxidation state of N is +4
Answer 4: Magnesium nitrate: this is a salt of the common acid, so it is named without including the oxidation state of the non-metal
Answer 5: Potassium sulfate: this is a salt of the common acid, so it is named without including the oxidation state of the non-metal
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





