- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记1.5.1 Shapes of Simple Molecules & Ions
Electron Pairs
- The valence shell electron pair repulsion theory (VSEPR) predicts the shape and bond angles of molecules
- Electrons are negatively charged and will repel other electrons when close to each other
- In a molecule, the bonding pairs of electrons will repel other electrons around the central atom forcing the molecule to adopt a shape in which these repulsive forces are minimised
- When determining the shape and bond angles of a molecule, the following VSEPR rules should be considered:
- Valence shell electrons are those electrons that are found in the outer shell
- Electron pairs repel each other as they have the same charge
- Lone pair electrons repel each other more than bonded pairs
- Repulsion between multiple and single bonds is treated the same as for repulsion between single bonds
- Repulsion between pairs of double bonds are greater
- The most stable shape is adopted to minimize the repulsion forces
- Different types of electron pairs have different repulsive forces
- Lone pairs of electrons have a more concentrated electron charge cloud than bonding pairs of electrons
- The cloud charges are wider and closer to the central atom’s nucleus
- The order of repulsion is therefore: lone pair – lone pair > lone pair – bond pair > bond pair – bond pair
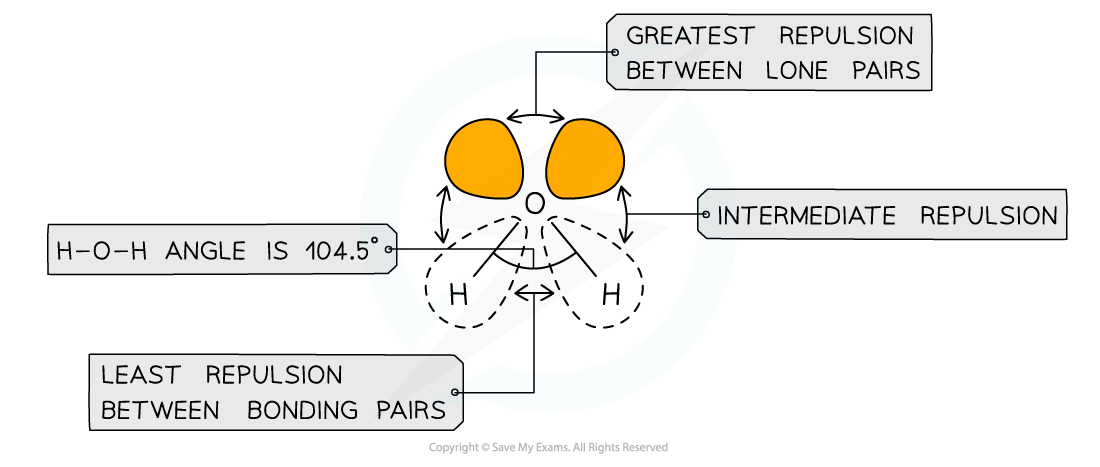
Different types of electron pairs have different repulsive forces
Shapes of Molecules & Ions
- Molecules can adapt the following shapes and bond angles:
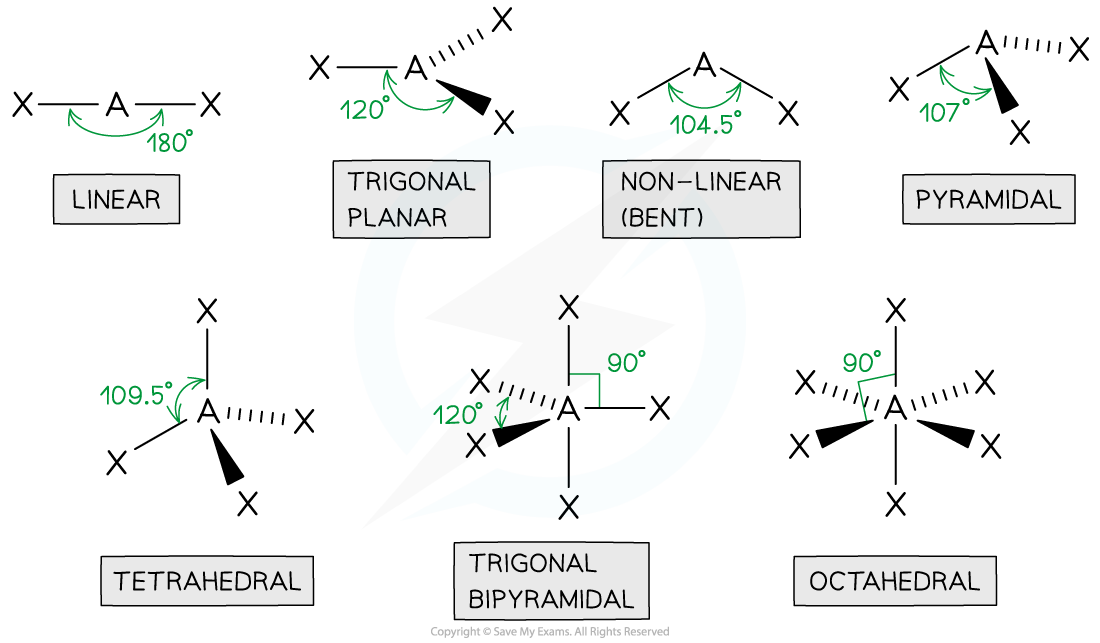
Molecules of different shapes can adapt with their corresponding bond angles
Examples
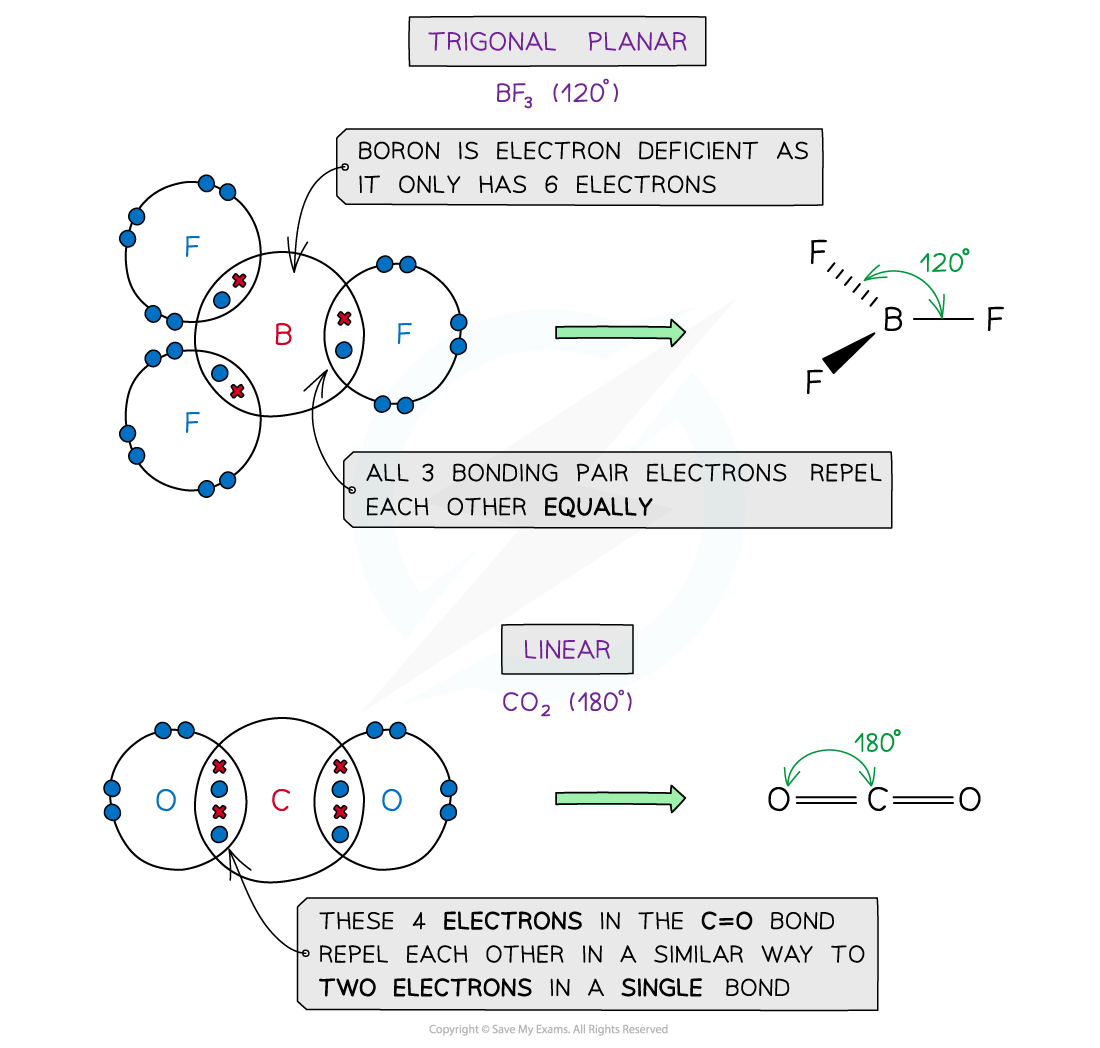
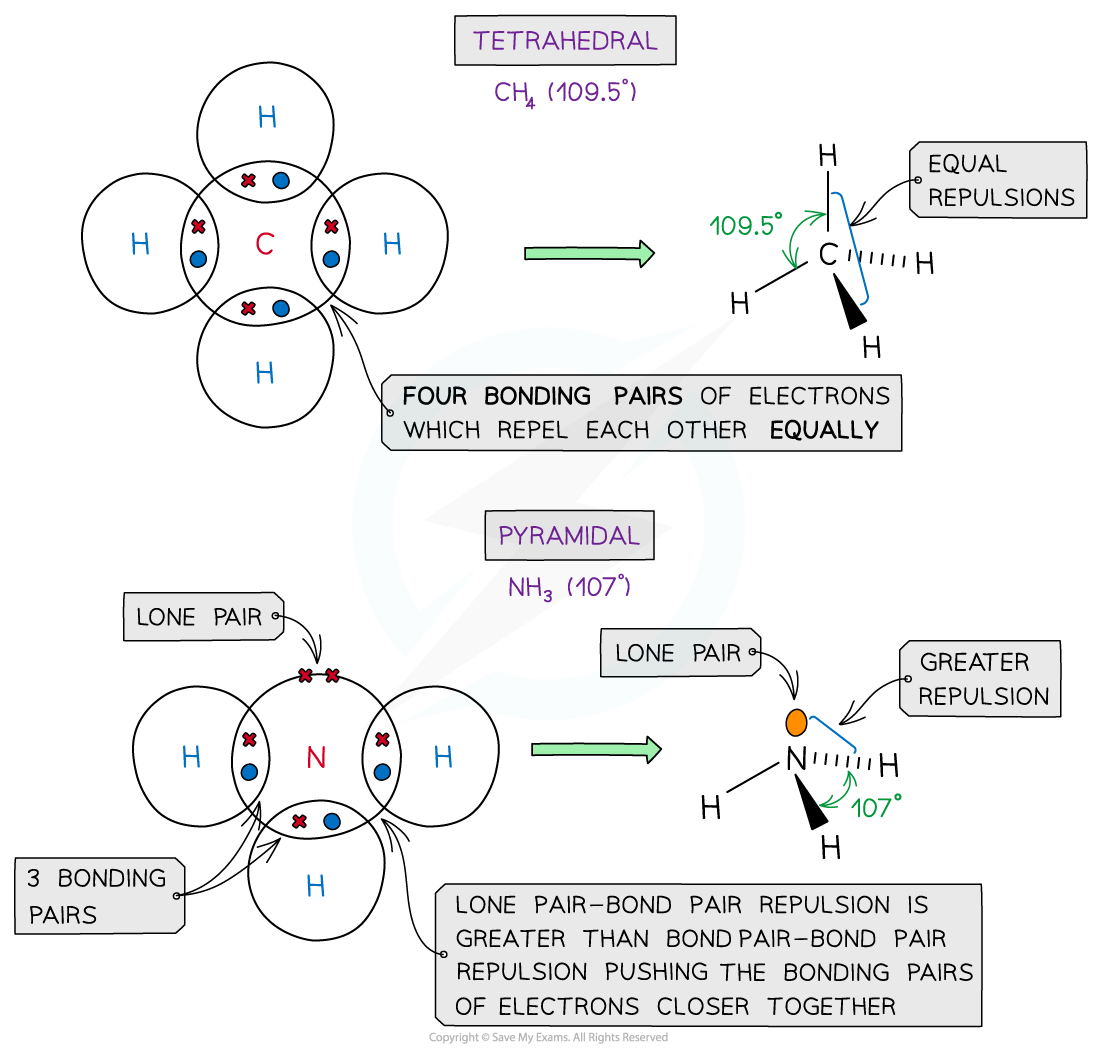
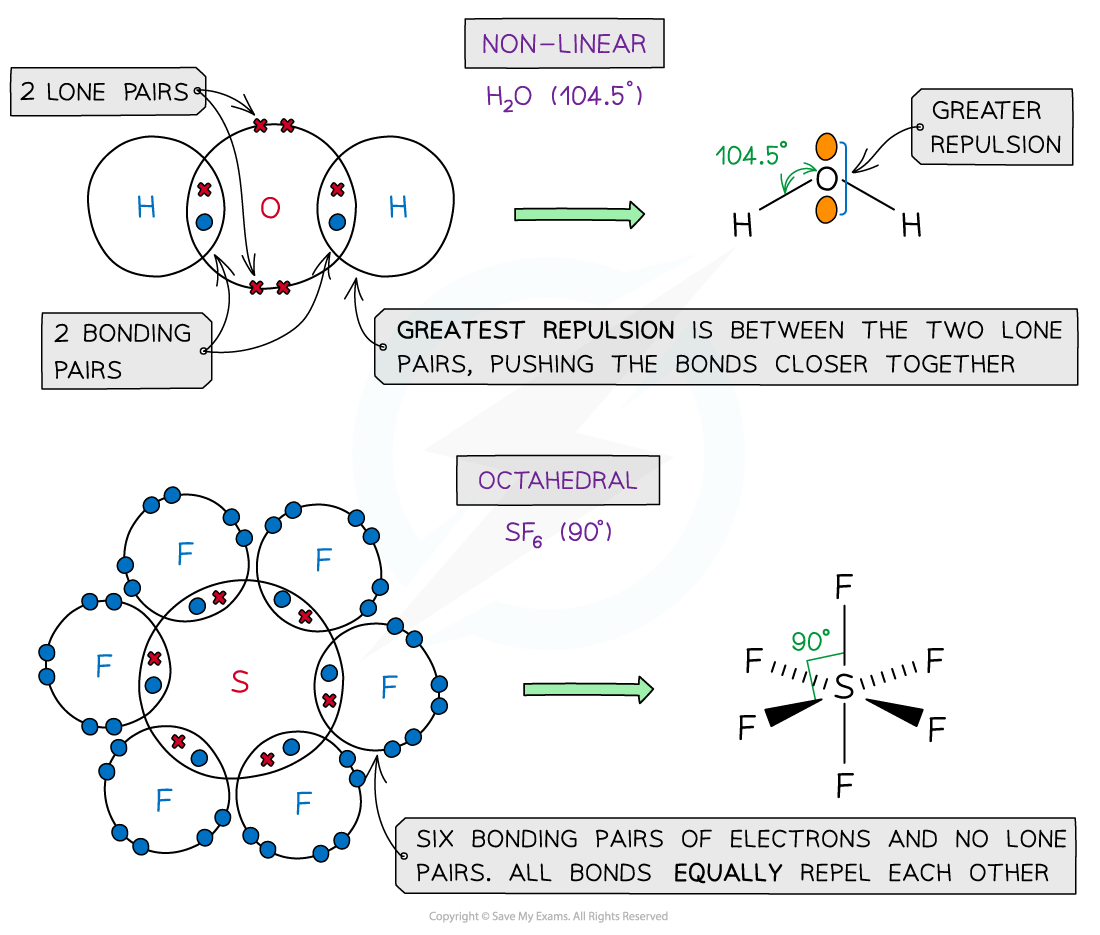
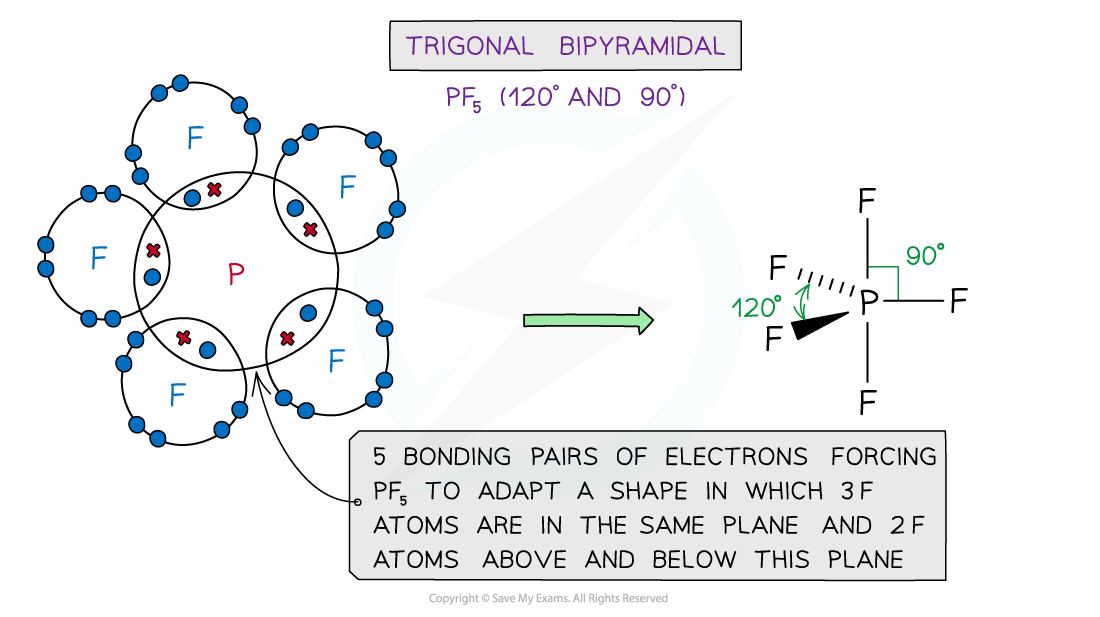
Examples of molecules with different shapes and bond angles
VSEPR & shapes of moleculesDraw the shape of the following molecules:
- Phosphorus(V) chloride
- N(CH3)3
- CCl4
Answer 1:
-
- Phosphorus is in group 15, so has 5 valence electrons; Cl is in group 17, so has 17 valence electrons
- All 5 electrons are used to form covalent bonds with Cl and there are no lone pairs
- This gives a trigonal (or triangular) bipyramidal shape:
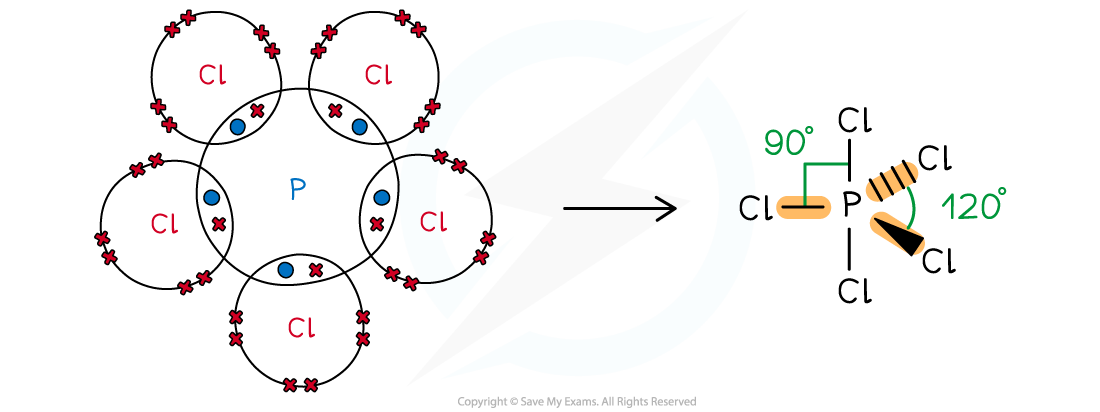
Phosphorus pentachloride or phosphorus (V) chloride
Answer 2:
-
- Nitrogen is in group 15, so has 5 valence electrons; carbon is in group 14, so has 4 valence electrons, 3 of which are already used in the covalent bonds with hydrogen
- Three of the valence electrons in N are used to form bonding pairs, so there is one lone pair left
- N(CH3)3 has a triangular pyramid shape:

Trimethylamine
Answer 3:
-
- Carbon is in group 14, so has 4 valence electrons; chlorine is in group 17, so has 7 valence electrons
- All four valence electrons are use to bond with chlorine and there are no lone pairs
- The shape of CCl4 is tetrahedral
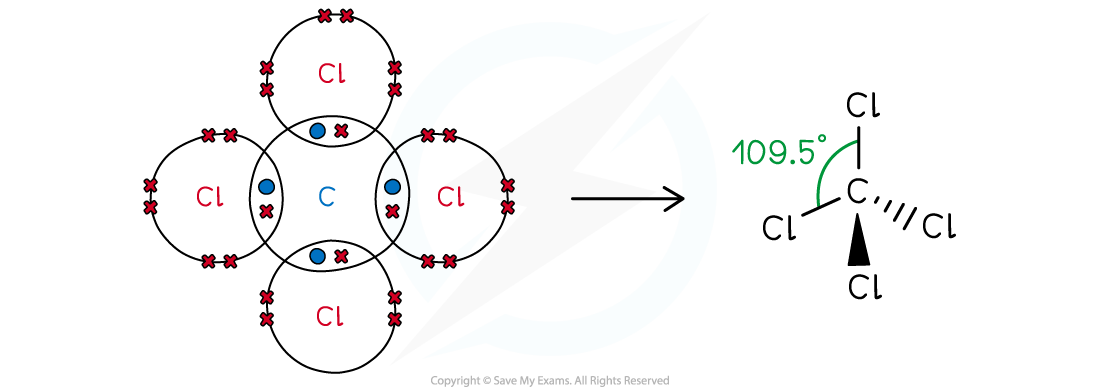
Tetrachloromethane
转载自savemyexams
站内搜索
竞赛真题免费下载(点击下载)
在线登记
最新发布
© 2024. All Rights Reserved. 沪ICP备2023009024号-1





